EGFR Inhibitors in Colorectal Cancer John L Marshall
EGFR Inhibitors in Colorectal Cancer John L. Marshall, MD Lombardi Comprehensive Cancer Center
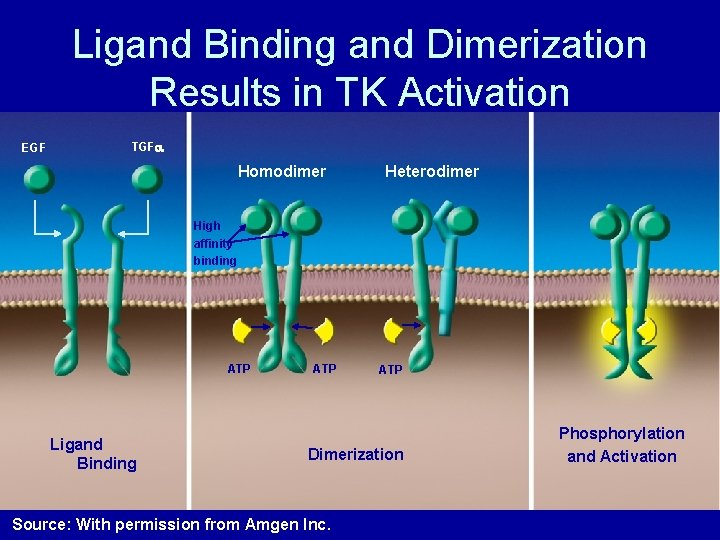
Ligand Binding and Dimerization Results in TK Activation EGF TGF Homodimer Heterodimer High affinity binding ATP Ligand Binding ATP Dimerization Source: With permission from Amgen Inc. Phosphorylation and Activation

EGFR Activation and Signaling Pathways Ligand EGFr dimer Signal Adapters and Enzymes Grb-2 Shc SOS Grb-2 P 13 K MAPK = mitogen-activated protein kinase P 13 k = phosphatidylinositol 3 -kinase m. TOR Transcription Factors SOS PTEN Raf Akt FKHR MEK 1/2 GSK-3 Source: With permission from Amgen Inc. BAD MAPK p 27 Jun FOS Myc Ras Cyclin D-1 Signal Cascade

EGFR Activation Mediates Several Processes EGFr Activation Tumor Spread of cancer cells Blood Vessel Metastatic Spread Cell Survival Tumor Angiogenesis Proliferation Source: With permission from Amgen Inc. Blood Vessel
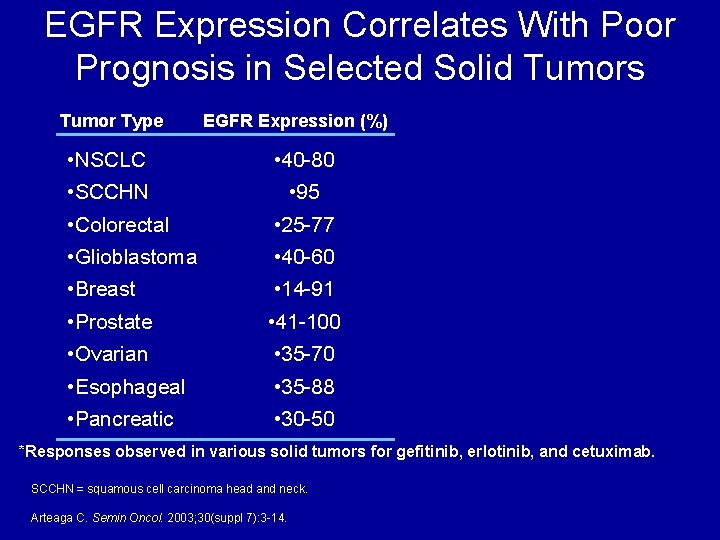
EGFR Expression Correlates With Poor Prognosis in Selected Solid Tumors Tumor Type EGFR Expression (%) • NSCLC • 40 -80 • SCCHN • 95 • Colorectal • 25 -77 • Glioblastoma • 40 -60 • Breast • 14 -91 • Prostate • 41 -100 • Ovarian • 35 -70 • Esophageal • 35 -88 • Pancreatic • 30 -50 *Responses observed in various solid tumors for gefitinib, erlotinib, and cetuximab. SCCHN = squamous cell carcinoma head and neck. Arteaga C. Semin Oncol. 2003; 30(suppl 7): 3 -14.
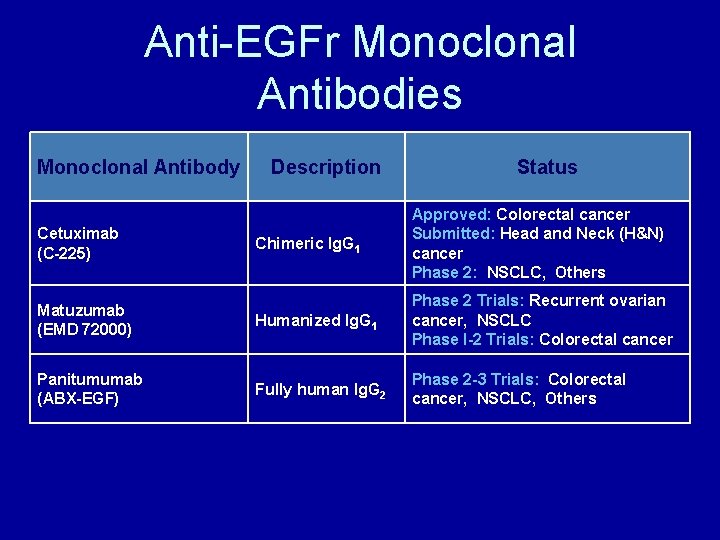
Anti-EGFr Monoclonal Antibodies Monoclonal Antibody Description Status Chimeric Ig. G 1 Approved: Colorectal cancer Submitted: Head and Neck (H&N) cancer Phase 2: NSCLC, Others Matuzumab (EMD 72000) Humanized Ig. G 1 Phase 2 Trials: Recurrent ovarian cancer, NSCLC Phase I-2 Trials: Colorectal cancer Panitumumab (ABX-EGF) Fully human Ig. G 2 Phase 2 -3 Trials: Colorectal cancer, NSCLC, Others Cetuximab (C-225) Mendelsohn J. J Clin Oncol. 2002; 20(suppl 18): 1 s-13 s. Tewes M, et al. Proc Am Soc Clin Oncol. 2002. Abstract 378. Sridhar SS, et al. Lancet Oncol. 2003; 4: 397 -406. Vanhoefer U, et al. J Clin Onc 2004; 22(1): 175 -184 www. clinicaltrials. gov
Properties of Cetuximab · Ig. G 1 MAb (chimerized) · Binds specifically to EGFR and its heterodimers · Binds to EGFR with high affinity (Kd = 2. 0 x 10– 10 M): 1 log higher than the natural ligand · Following the recommended dose regimen (400 mg/m 2 initial dose/250 mg/m 2 weekly dose), the mean half-life was 114 hours (range 75 -188 hours) · Competitively inhibits ligand binding to EGFR · Stimulates receptor internalization · Blocks receptor dimerization, tyrosine kinase phosphorylation, and signal transduction Shitara K, et al. Cancer Immunol Immunother. 1993; 36: 373 -380. Lo. Buglio AF, et al. Proc Natl Acad Sci U S A. 1989; 86: 4220 -4224. ERBITUX Package Insert, June 2004. Data on file. Im. Clone Systems Incorporated and Bristol-Myers Squibb Company; 2004.
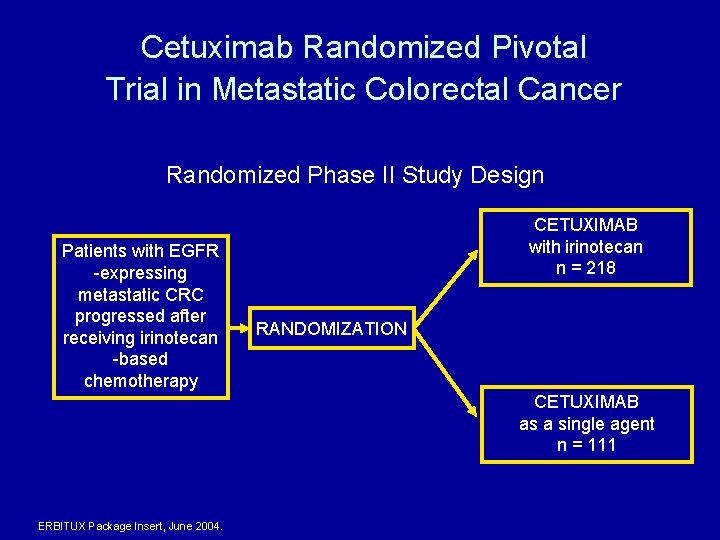
Cetuximab Randomized Pivotal Trial in Metastatic Colorectal Cancer Randomized Phase II Study Design Patients with EGFR -expressing metastatic CRC progressed after receiving irinotecan -based chemotherapy CETUXIMAB with irinotecan n = 218 RANDOMIZATION CETUXIMAB as a single agent n = 111 ERBITUX Package Insert, June 2004.
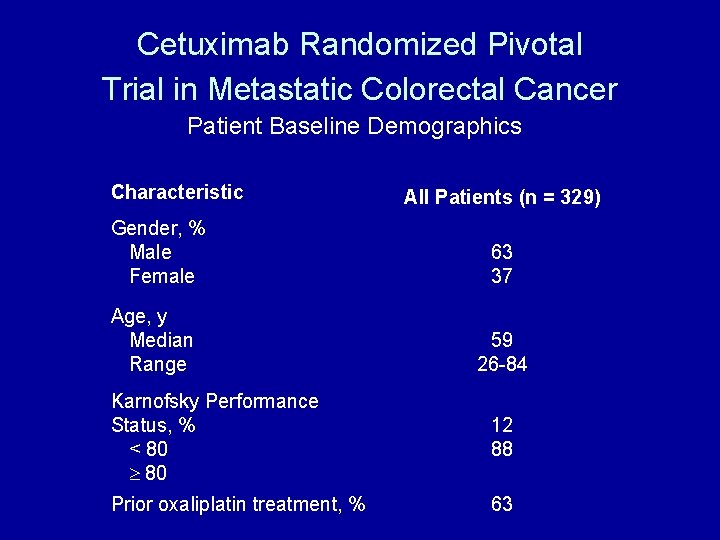
Cetuximab Randomized Pivotal Trial in Metastatic Colorectal Cancer Patient Baseline Demographics Characteristic All Patients (n = 329) Gender, % Male Female 63 37 Age, y Median Range 59 26 -84 Karnofsky Performance Status, % < 80 12 88 Prior oxaliplatin treatment, % 63 ERBITUX Package Insert, June 2004.
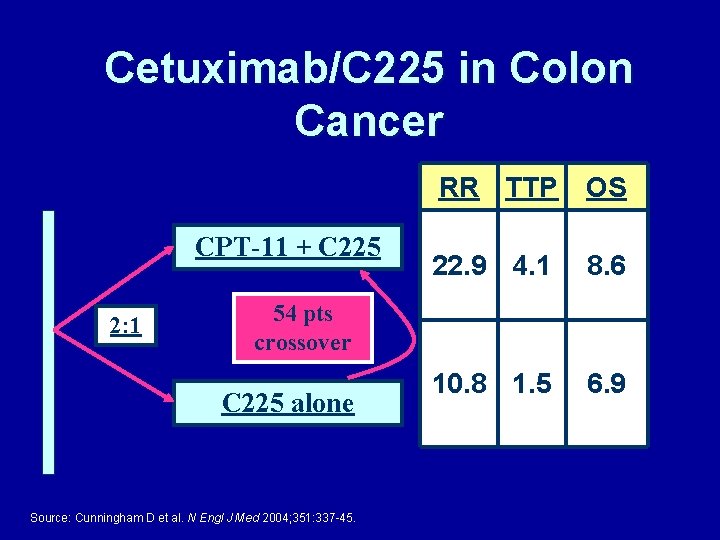
Cetuximab/C 225 in Colon Cancer CPT-11 + C 225 2: 1 RR TTP OS 22. 9 4. 1 8. 6 10. 8 1. 5 6. 9 54 pts crossover C 225 alone Source: Cunningham D et al. N Engl J Med 2004; 351: 337 -45.
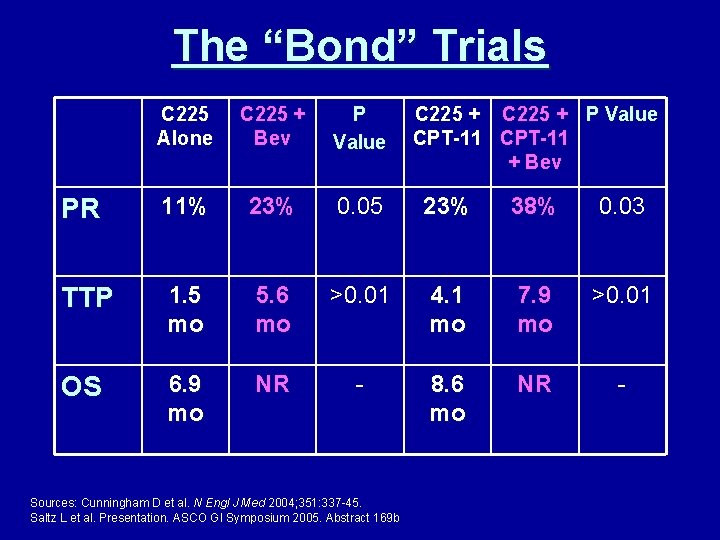
The “Bond” Trials C 225 Alone C 225 + Bev P Value PR 11% 23% 0. 05 23% 38% 0. 03 TTP 1. 5 mo 5. 6 mo >0. 01 4. 1 mo 7. 9 mo >0. 01 OS 6. 9 mo NR - 8. 6 mo NR - Sources: Cunningham D et al. N Engl J Med 2004; 351: 337 -45. Saltz L et al. Presentation. ASCO GI Symposium 2005. Abstract 169 b C 225 + P Value CPT-11 + Bev
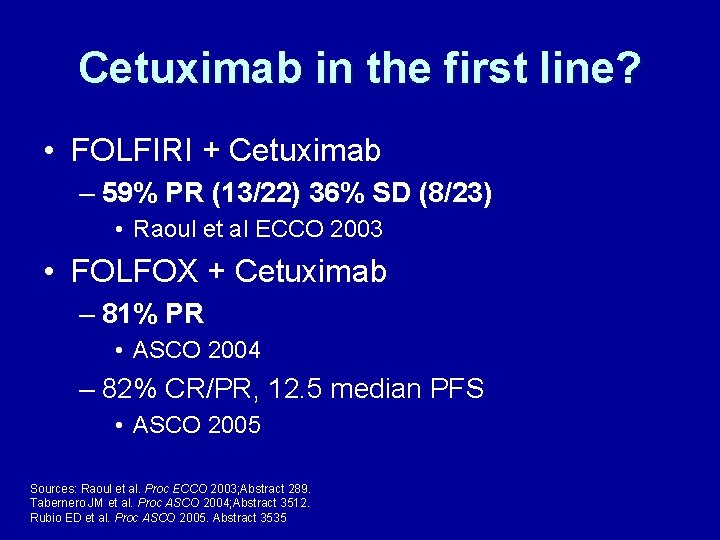
Cetuximab in the first line? • FOLFIRI + Cetuximab – 59% PR (13/22) 36% SD (8/23) • Raoul et al ECCO 2003 • FOLFOX + Cetuximab – 81% PR • ASCO 2004 – 82% CR/PR, 12. 5 median PFS • ASCO 2005 Sources: Raoul et al. Proc ECCO 2003; Abstract 289. Tabernero JM et al. Proc ASCO 2004; Abstract 3512. Rubio ED et al. Proc ASCO 2005. Abstract 3535
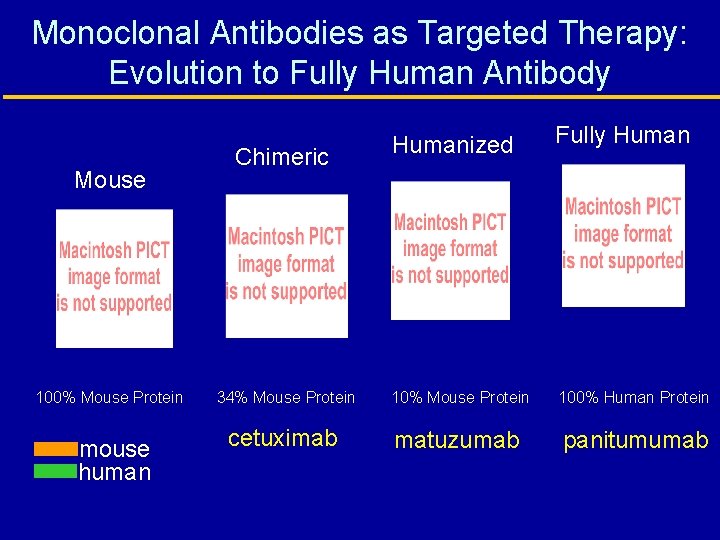
Monoclonal Antibodies as Targeted Therapy: Evolution to Fully Human Antibody Mouse 100% Mouse Protein mouse human Chimeric Humanized Fully Human 34% Mouse Protein 100% Human Protein cetuximab matuzumab panitumumab
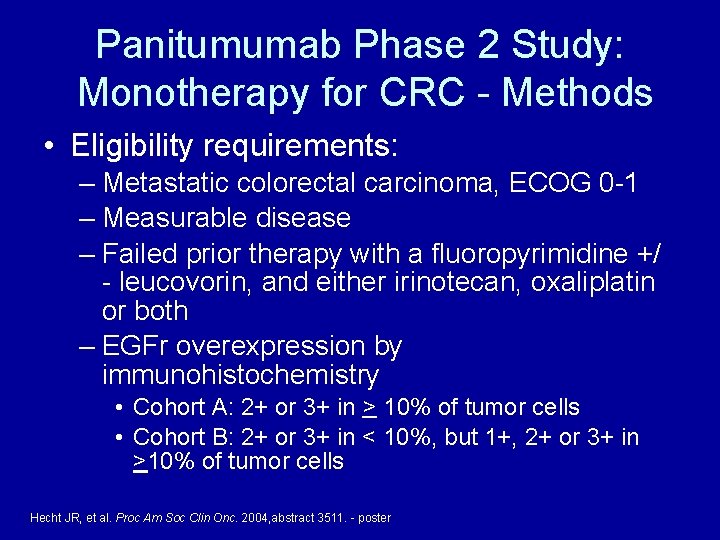
Panitumumab Phase 2 Study: Monotherapy for CRC - Methods • Eligibility requirements: – Metastatic colorectal carcinoma, ECOG 0 -1 – Measurable disease – Failed prior therapy with a fluoropyrimidine +/ - leucovorin, and either irinotecan, oxaliplatin or both – EGFr overexpression by immunohistochemistry • Cohort A: 2+ or 3+ in > 10% of tumor cells • Cohort B: 2+ or 3+ in < 10%, but 1+, 2+ or 3+ in >10% of tumor cells Hecht JR, et al. Proc Am Soc Clin Onc. 2004, abstract 3511. - poster

Panitumumab Phase 2 Study: Monotherapy for CRC - Methods • Dose: 2. 5 mg/kg – Infused over 1 hour – No loading dose – Administered without premedication – Given weekly until disease progression or unacceptable toxicity • Assessments: – Toxicity assessed weekly – Tumor assessment every 8 weeks Hecht JR, et al. Proc Am Soc Clin Onc. 2004, abstract 3511. - poster
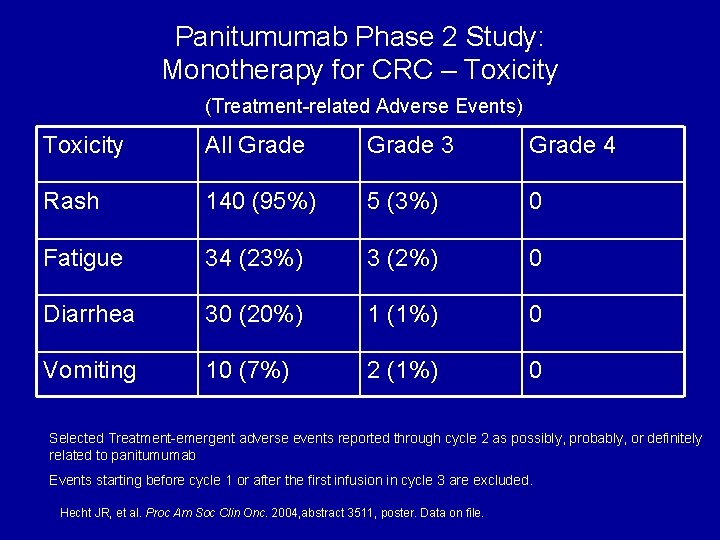
Panitumumab Phase 2 Study: Monotherapy for CRC – Toxicity (Treatment-related Adverse Events) Toxicity All Grade 3 Grade 4 Rash 140 (95%) 5 (3%) 0 Fatigue 34 (23%) 3 (2%) 0 Diarrhea 30 (20%) 1 (1%) 0 Vomiting 10 (7%) 2 (1%) 0 Selected Treatment-emergent adverse events reported through cycle 2 as possibly, probably, or definitely related to panitumumab Events starting before cycle 1 or after the first infusion in cycle 3 are excluded. Hecht JR, et al. Proc Am Soc Clin Onc. 2004, abstract 3511, poster. Data on file.
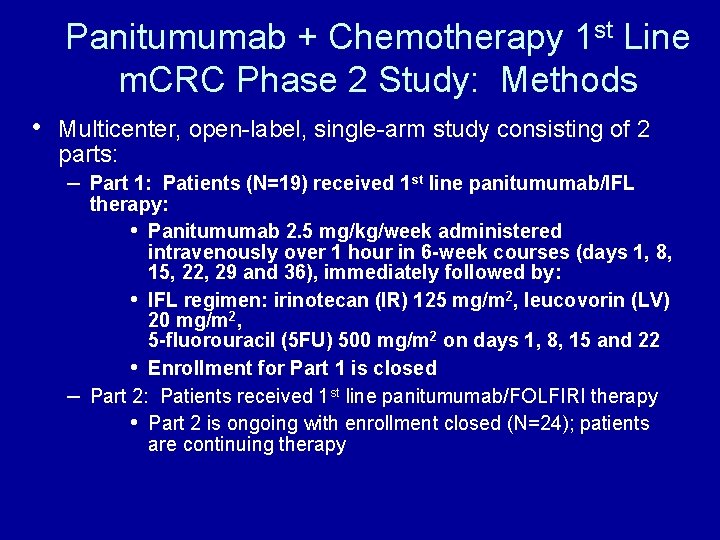
Panitumumab + Chemotherapy 1 st Line m. CRC Phase 2 Study: Methods • Multicenter, open-label, single-arm study consisting of 2 parts: – Part 1: Patients (N=19) received 1 st line panitumumab/IFL – therapy: • Panitumumab 2. 5 mg/kg/week administered intravenously over 1 hour in 6 -week courses (days 1, 8, 15, 22, 29 and 36), immediately followed by: • IFL regimen: irinotecan (IR) 125 mg/m 2, leucovorin (LV) 20 mg/m 2, 5 -fluorouracil (5 FU) 500 mg/m 2 on days 1, 8, 15 and 22 • Enrollment for Part 1 is closed Part 2: Patients received 1 st line panitumumab/FOLFIRI therapy • Part 2 is ongoing with enrollment closed (N=24); patients are continuing therapy Berlin J, et al. ESMO 2004. a 265
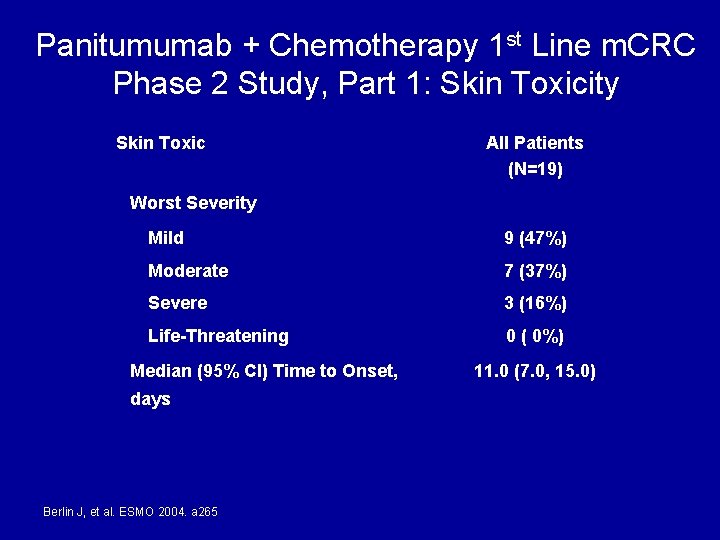
Panitumumab + Chemotherapy 1 st Line m. CRC Phase 2 Study, Part 1: Skin Toxicity Skin Toxic All Patients (N=19) Worst Severity Mild 9 (47%) Moderate 7 (37%) Severe 3 (16%) Life-Threatening 0 ( 0%) Median (95% CI) Time to Onset, days Berlin J, et al. ESMO 2004. a 265 11. 0 (7. 0, 15. 0)
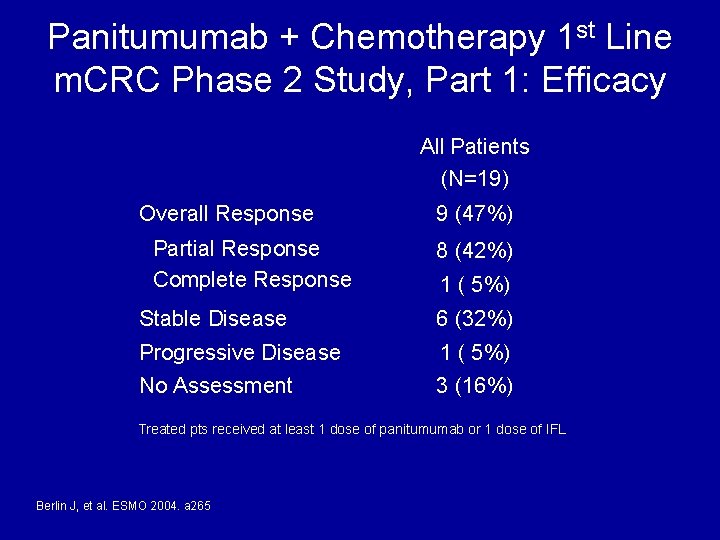
Panitumumab + Chemotherapy 1 st Line m. CRC Phase 2 Study, Part 1: Efficacy All Patients (N=19) Overall Response Partial Response Complete Response 9 (47%) 8 (42%) 1 ( 5%) Stable Disease 6 (32%) Progressive Disease 1 ( 5%) No Assessment 3 (16%) Treated pts received at least 1 dose of panitumumab or 1 dose of IFL Berlin J, et al. ESMO 2004. a 265
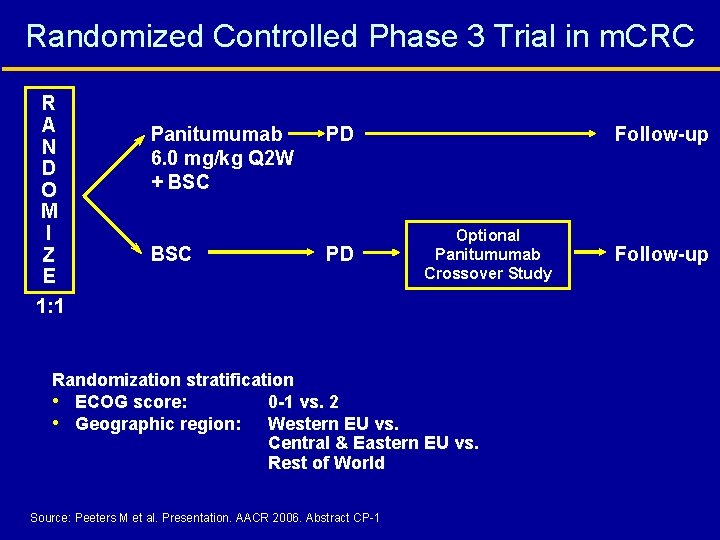
Randomized Controlled Phase 3 Trial in m. CRC R A N D O M I Z E 1: 1 Panitumumab 6. 0 mg/kg Q 2 W + BSC PD PD Follow-up Optional Panitumumab Crossover Study Randomization stratification • ECOG score: 0 -1 vs. 2 • Geographic region: Western EU vs. Central & Eastern EU vs. Rest of World Source: Peeters M et al. Presentation. AACR 2006. Abstract CP-1 Follow-up
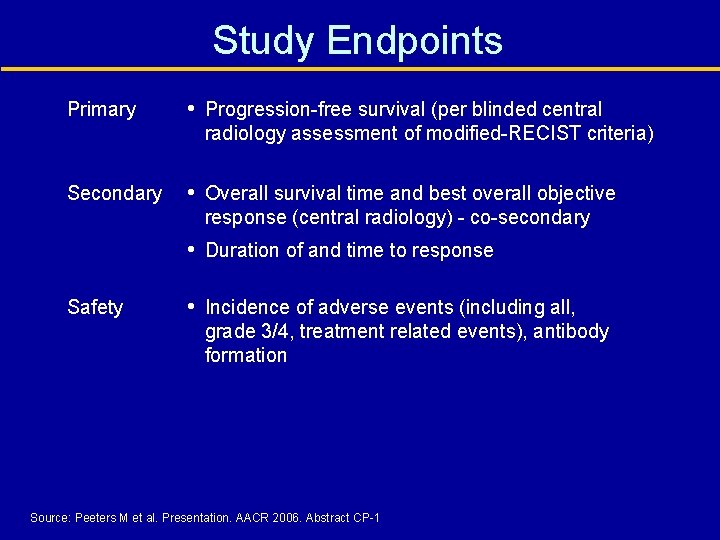
Study Endpoints Primary • Progression-free survival (per blinded central radiology assessment of modified-RECIST criteria) Secondary • Overall survival time and best overall objective response (central radiology) - co-secondary • Duration of and time to response Safety • Incidence of adverse events (including all, grade 3/4, treatment related events), antibody formation Source: Peeters M et al. Presentation. AACR 2006. Abstract CP-1
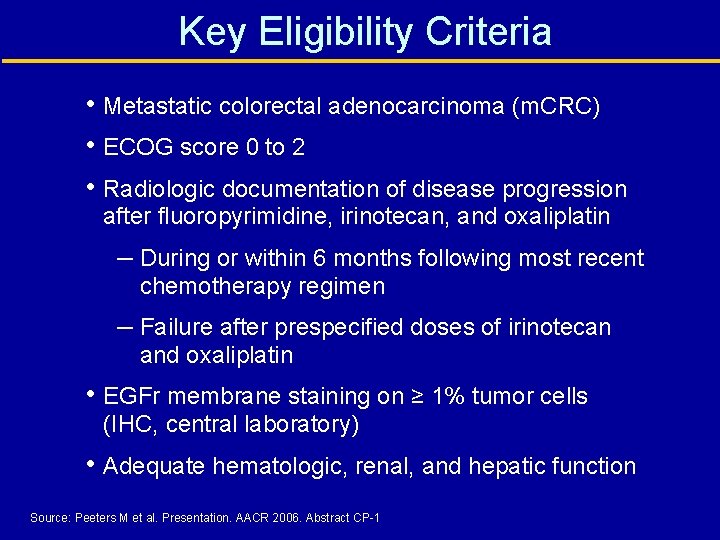
Key Eligibility Criteria • Metastatic colorectal adenocarcinoma (m. CRC) • ECOG score 0 to 2 • Radiologic documentation of disease progression after fluoropyrimidine, irinotecan, and oxaliplatin – During or within 6 months following most recent chemotherapy regimen – Failure after prespecified doses of irinotecan and oxaliplatin • EGFr membrane staining on ≥ 1% tumor cells (IHC, central laboratory) • Adequate hematologic, renal, and hepatic function Source: Peeters M et al. Presentation. AACR 2006. Abstract CP-1
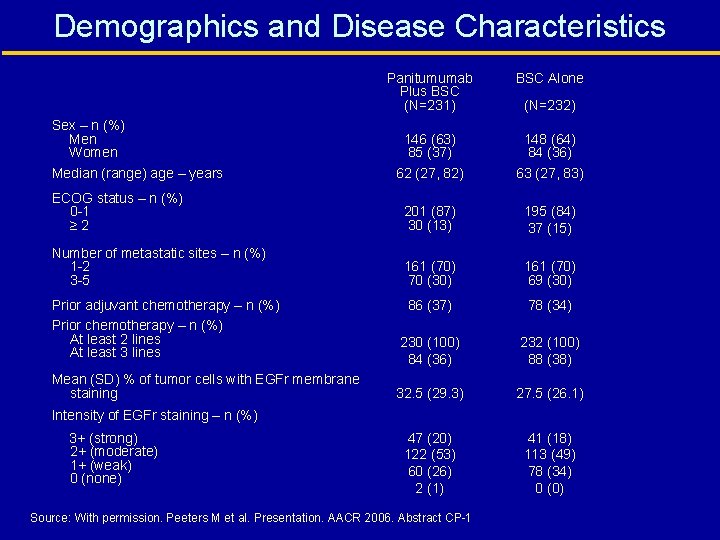
Demographics and Disease Characteristics Panitumumab Plus BSC (N=231) BSC Alone 146 (63) 85 (37) 62 (27, 82) 148 (64) 84 (36) 63 (27, 83) ECOG status – n (%) 0 -1 ≥ 2 201 (87) 30 (13) 195 (84) 37 (15) Number of metastatic sites – n (%) 1 -2 3 -5 161 (70) 70 (30) 161 (70) 69 (30) Sex – n (%) Men Women Median (range) age – years (N=232) Prior adjuvant chemotherapy – n (%) Prior chemotherapy – n (%) At least 2 lines At least 3 lines 86 (37) 78 (34) 230 (100) 84 (36) 232 (100) 88 (38) Mean (SD) % of tumor cells with EGFr membrane staining 32. 5 (29. 3) 27. 5 (26. 1) 47 (20) 122 (53) 60 (26) 2 (1) 41 (18) 113 (49) 78 (34) 0 (0) Intensity of EGFr staining – n (%) 3+ (strong) 2+ (moderate) 1+ (weak) 0 (none) Source: With permission. Peeters M et al. Presentation. AACR 2006. Abstract CP-1
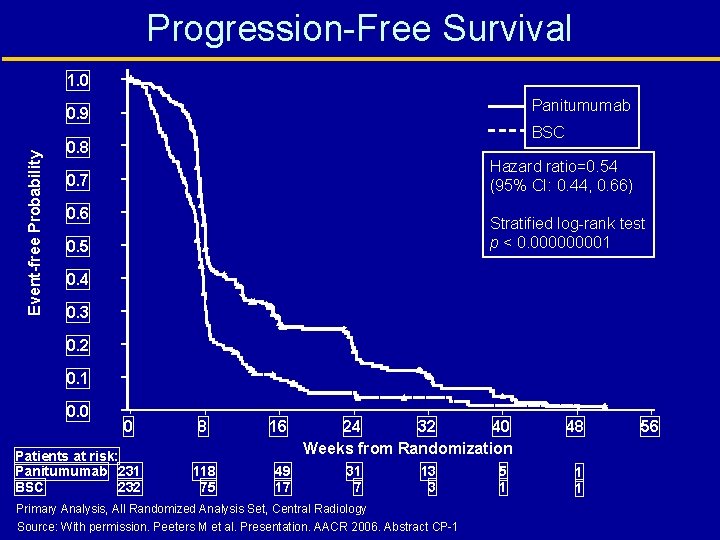
Progression-Free Survival 1. 0 Panitumumab Event-free Probability 0. 9 BSC 0. 8 Hazard ratio=0. 54 (95% CI: 0. 44, 0. 66) 0. 7 0. 6 Stratified log-rank test p < 0. 00001 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 Patients at risk: Panitumumab 231 BSC 232 8 118 75 16 49 17 24 32 40 Weeks from Randomization 31 7 13 3 Primary Analysis, All Randomized Analysis Set, Central Radiology Source: With permission. Peeters M et al. Presentation. AACR 2006. Abstract CP-1 5 1 48 1 1 56
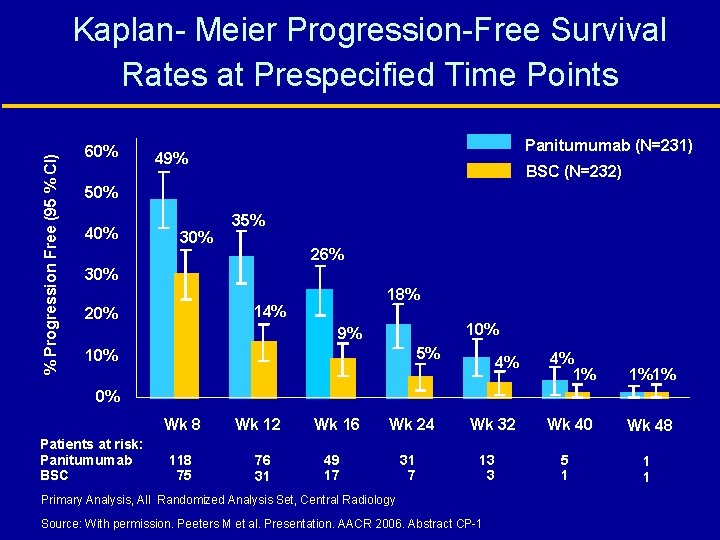
% Progression Free (95 % CI) Kaplan- Meier Progression-Free Survival Rates at Prespecified Time Points 60% Panitumumab (N=231) 49% BSC (N=232) 50% 40% 35% 26% 30% 18% 14% 20% 10% 9% 5% 10% 4% 4% 1% 1%1% Wk 32 Wk 40 Wk 48 0% Wk 8 Patients at risk: Panitumumab BSC 118 75 Wk 12 76 31 Wk 16 Wk 24 49 17 31 7 13 3 Primary Analysis, All Randomized Analysis Set, Central Radiology Source: With permission. Peeters M et al. Presentation. AACR 2006. Abstract CP-1 5 1 1 1
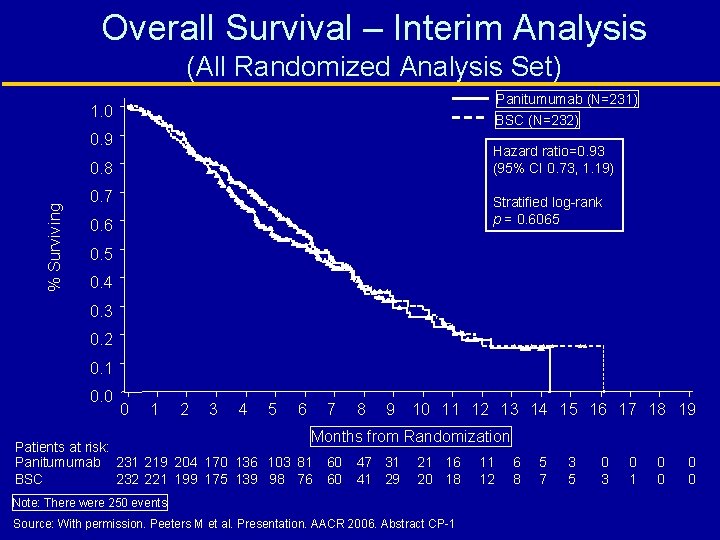
Overall Survival – Interim Analysis (All Randomized Analysis Set) Panitumumab (N=231) 1. 0 BSC (N=232) 0. 9 Hazard ratio=0. 93 (95% CI 0. 73, 1. 19) % Surviving 0. 8 0. 7 Stratified log-rank p = 0. 6065 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Months from Randomization Patients at risk: Panitumumab 231 219 204 170 136 103 81 60 232 221 199 175 139 98 76 60 BSC 47 31 41 29 21 16 20 18 Note: There were 250 events Source: With permission. Peeters M et al. Presentation. AACR 2006. Abstract CP-1 11 12 6 8 5 7 3 5 0 3 0 1 0 0
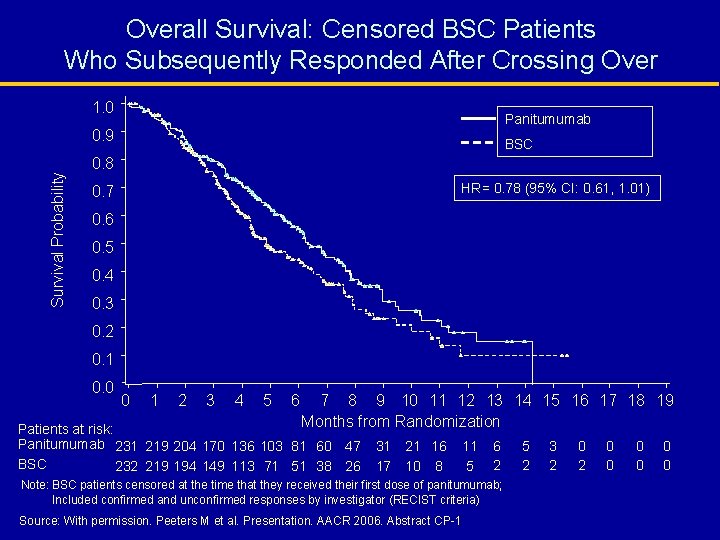
Overall Survival: Censored BSC Patients Who Subsequently Responded After Crossing Over 1. 0 Panitumumab Survival Probability 0. 9 BSC 0. 8 HR= 0. 78 (95% CI: 0. 61, 1. 01) 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Months from Randomization Patients at risk: Panitumumab 231 219 204 170 136 103 81 60 47 BSC 232 219 194 149 113 71 51 38 26 31 21 16 17 10 8 11 5 6 2 Note: BSC patients censored at the time that they received their first dose of panitumumab; Included confirmed and unconfirmed responses by investigator (RECIST criteria) Source: With permission. Peeters M et al. Presentation. AACR 2006. Abstract CP-1 5 2 3 2 0 0 0 0
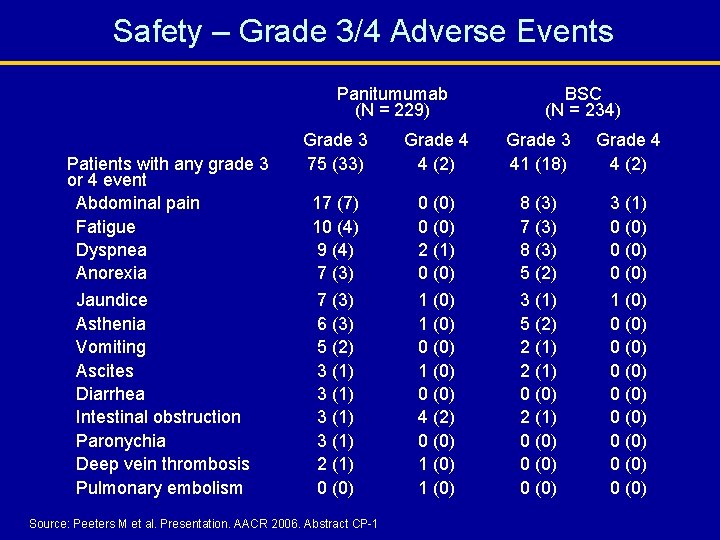
Safety – Grade 3/4 Adverse Events Panitumumab (N = 229) Patients with any grade 3 or 4 event Abdominal pain Fatigue Dyspnea Anorexia Jaundice Asthenia Vomiting Ascites Diarrhea Intestinal obstruction Paronychia Deep vein thrombosis Pulmonary embolism BSC (N = 234) Grade 3 75 (33) Grade 4 4 (2) Grade 3 41 (18) Grade 4 4 (2) 17 (7) 10 (4) 9 (4) 7 (3) 6 (3) 5 (2) 3 (1) 2 (1) 0 (0) 2 (1) 0 (0) 1 (0) 0 (0) 4 (2) 0 (0) 1 (0) 8 (3) 7 (3) 8 (3) 5 (2) 3 (1) 5 (2) 2 (1) 0 (0) 0 (0) 3 (1) 0 (0) 1 (0) 0 (0) 0 (0) Med. DRA version 8. 0 preferred terms; graded per NCI CTCAE version 2. 0 Source: Peeters M et al. Presentation. AACR 2006. Abstract CP-1
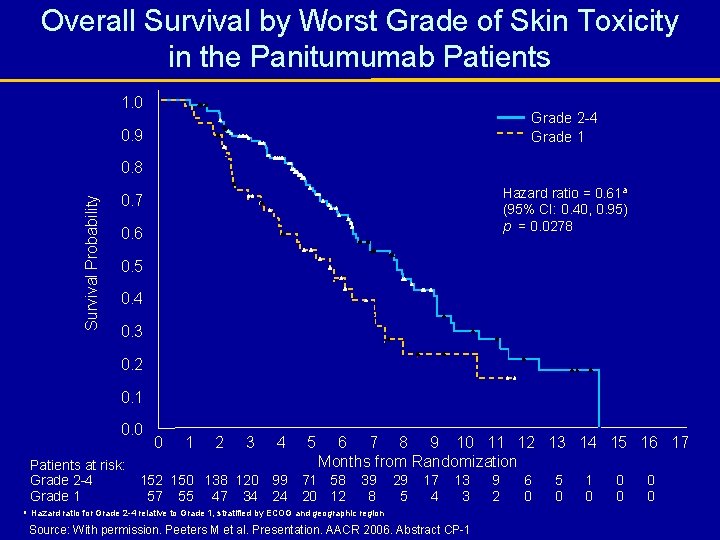
Overall Survival by Worst Grade of Skin Toxicity in the Panitumumab Patients 1. 0 Grade 2 -4 Grade 1 0. 9 Survival Probability 0. 8 Hazard ratio = 0. 61 a (95% CI: 0. 40, 0. 95) p = 0. 0278 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Months from Randomization Patients at risk: Grade 2 -4 152 150 138 120 99 71 58 Grade 1 57 55 47 34 24 20 12 a 39 8 29 5 17 4 13 3 Hazard ratio for Grade 2 -4 relative to Grade 1, stratified by ECOG and geographic region Source: With permission. Peeters M et al. Presentation. AACR 2006. Abstract CP-1 9 2 6 0 5 0 1 0 0 0

Conclusions and Questions • EGFR antibodies are active in colon cancer • Skin rash toxicity is the biggest barrier to more widespread use • Approval(s) in “last line” therapy – Role in 1 st line or adjuvant to be determined
- Slides: 30