Effects of Recommending a Minimum Interval Schedule for












- Slides: 33

Effects of Recommending a Minimum Interval Schedule for DTa. P during a Statewide Pertussis Outbreak in Arizona Daniel Bronson-Lowe, Kimiko Gosney, Susan Goodykoontz, Kathy Fredrickson and Shoana Anderson Arizona Department of Health Services National Immunization Conference March 7, 2007 Infectious Disease Epidemiology Arizona Immunization Program

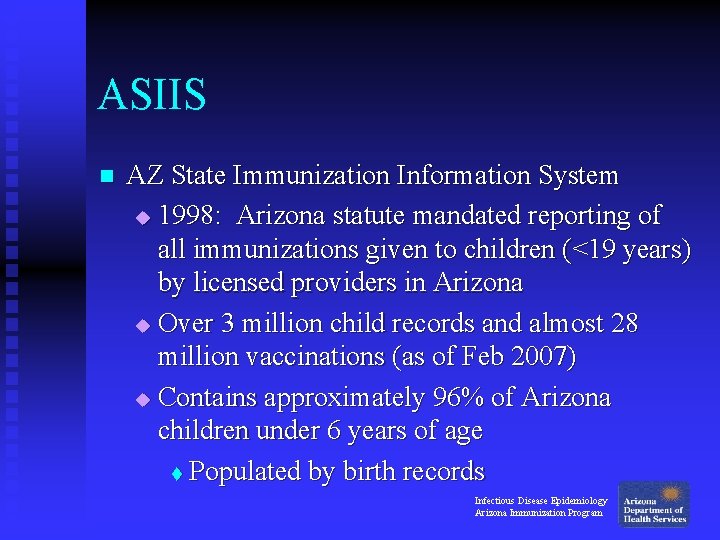
ASIIS n AZ State Immunization Information System u 1998: Arizona statute mandated reporting of all immunizations given to children (<19 years) by licensed providers in Arizona u Over 3 million child records and almost 28 million vaccinations (as of Feb 2007) u Contains approximately 96% of Arizona children under 6 years of age t Populated by birth records Infectious Disease Epidemiology Arizona Immunization Program

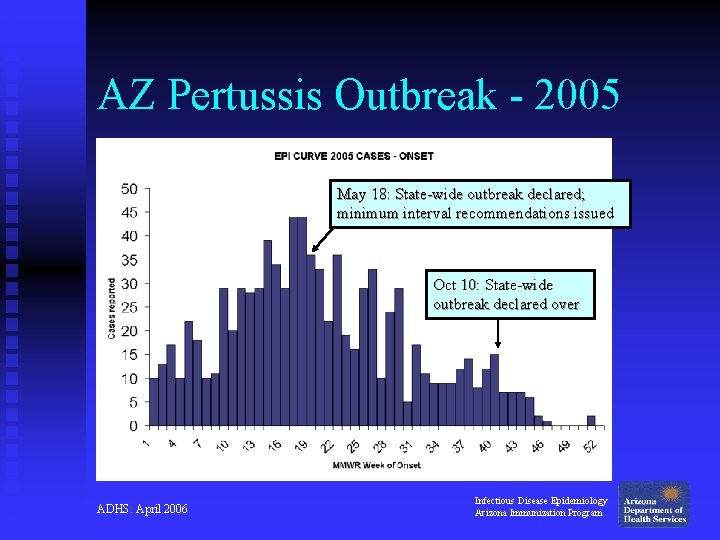
AZ Pertussis Outbreak - 2005 n n May 2005: Several Arizona counties noted increased levels of reported pertussis cases over the previous months, including middle/high school outbreaks May 18 th: State-wide outbreak declared Oct 10 th: State-wide outbreak declared over 832 pertussis cases were reported Infectious Disease Epidemiology Arizona Immunization Program

AZ Pertussis Outbreak - 2005 May 18: State-wide outbreak declared; minimum interval recommendations issued Oct 10: State-wide outbreak declared over ADHS: April 2006 Infectious Disease Epidemiology Arizona Immunization Program

Outbreak Recommendations n n Infant minimum interval DTa. P immunization schedule was recommended statewide u Relies on minimum age for first dose and minimum intervals for subsequent doses to get the first three doses of DTa. P to infants as early as possible Concerns were raised that this recommendation might disrupt the schedules of other vaccines normally given at the same time Infectious Disease Epidemiology Arizona Immunization Program

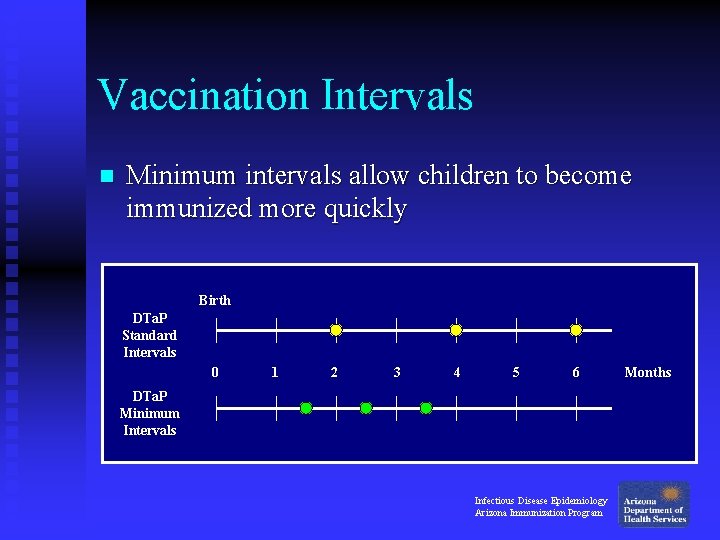
Vaccination Intervals n Minimum intervals allow children to become immunized more quickly Birth DTa. P Standard Intervals 0 1 2 3 4 5 6 DTa. P Minimum Intervals Infectious Disease Epidemiology Arizona Immunization Program Months

Objectives n Does administration of DTa. P on a minimum interval schedule have an adverse effect on the administration of recommended doses of: 1. DTa. P (diphtheria, tetanus, and pertussis) 2. IPV (inactivated polio vaccine) 1. 2. often given at the same visits as DTa. P can be given in combination with DTa. P PCV (pneumococcal conjugate vaccine) 3. 1. 2. often given at the same visits as DTa. P not given in combination Infectious with. Disease DTa. P Epidemiology Arizona Immunization Program

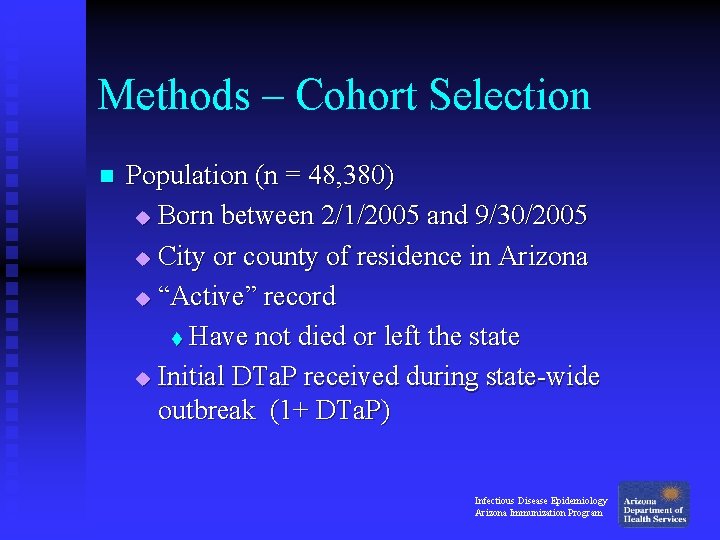
Methods – Cohort Selection n Population (n = 48, 380) u Born between 2/1/2005 and 9/30/2005 u City or county of residence in Arizona u “Active” record t Have not died or left the state u Initial DTa. P received during state-wide outbreak (1+ DTa. P) Infectious Disease Epidemiology Arizona Immunization Program

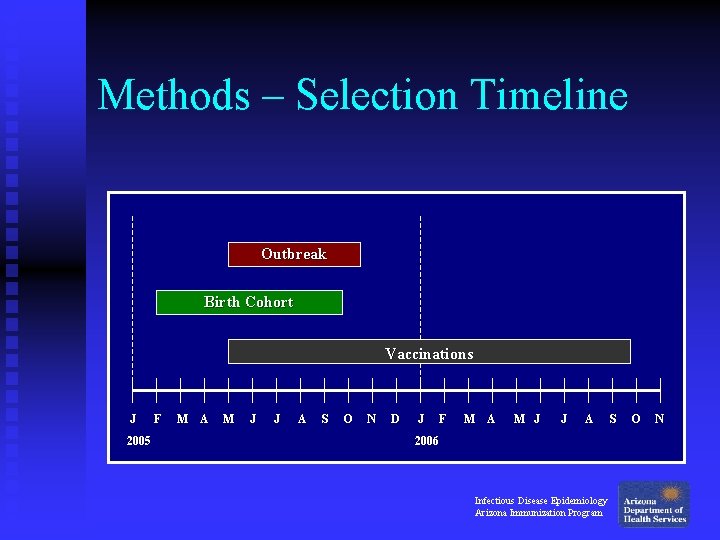
Methods – Selection Timeline Outbreak Birth Cohort Vaccinations J 2005 F M A M J J A S O N D J F M A M J J A 2006 Infectious Disease Epidemiology Arizona Immunization Program S O N

Methods – Vaccinations n n Algorithm designed to select the first 3 valid DTa. P doses (SAS 9. 1) u Validity defined by CDC Recommended Immunization Schedule Determined interval status u Minimum interval vs. standard t Based on CDC Recommended Immunization Schedule Infectious Disease Epidemiology Arizona Immunization Program

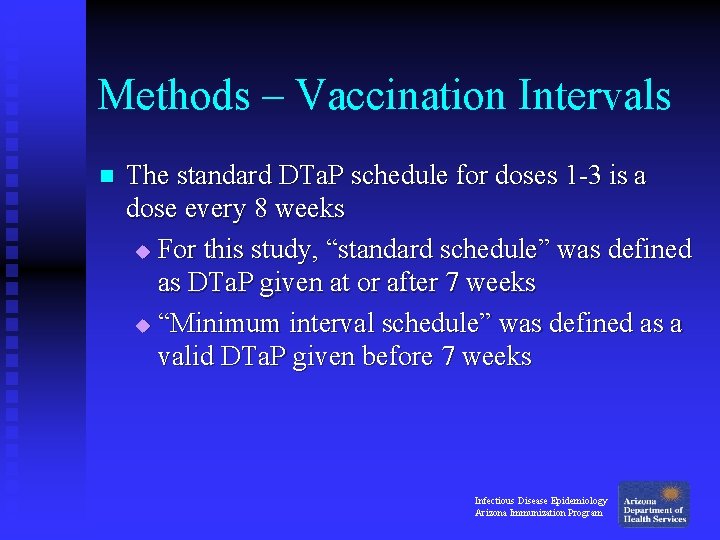
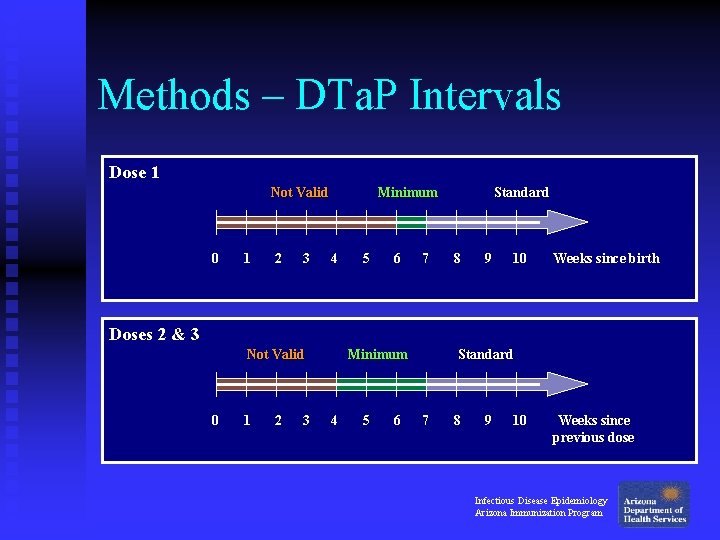
Methods – Vaccination Intervals n The standard DTa. P schedule for doses 1 -3 is a dose every 8 weeks u For this study, “standard schedule” was defined as DTa. P given at or after 7 weeks u “Minimum interval schedule” was defined as a valid DTa. P given before 7 weeks Infectious Disease Epidemiology Arizona Immunization Program

Methods – DTa. P Intervals Dose 1 Not Valid 0 1 2 3 Minimum 4 5 6 7 Standard 8 9 10 Weeks since birth Doses 2 & 3 Not Valid 0 1 2 3 Minimum 4 5 6 Standard 7 8 9 10 Weeks since previous dose Infectious Disease Epidemiology Arizona Immunization Program

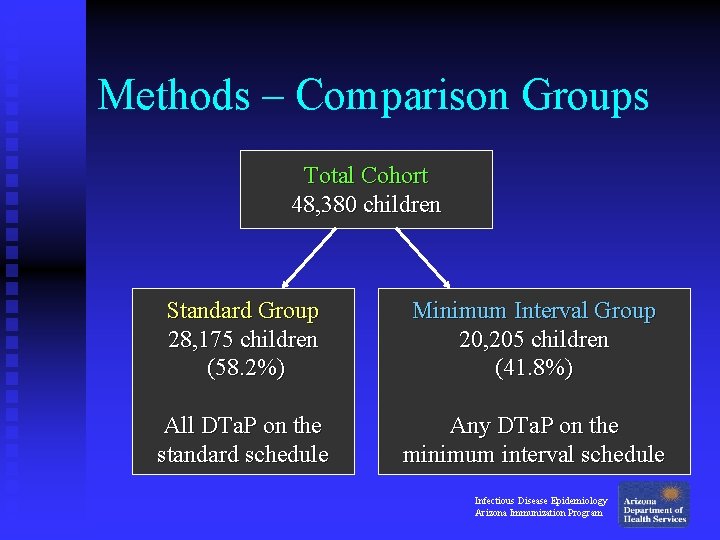
Methods – Comparison Groups Total Cohort 48, 380 children Standard Group 28, 175 children (58. 2%) Minimum Interval Group 20, 205 children (41. 8%) All DTa. P on the standard schedule Any DTa. P on the minimum interval schedule Infectious Disease Epidemiology Arizona Immunization Program

Objective 1 – DTa. P n Compare the Standard group with the Minimum Interval group u Completion rates of 3 doses of DTa. P u Mean age at the time of the third DTa. P dose Infectious Disease Epidemiology Arizona Immunization Program

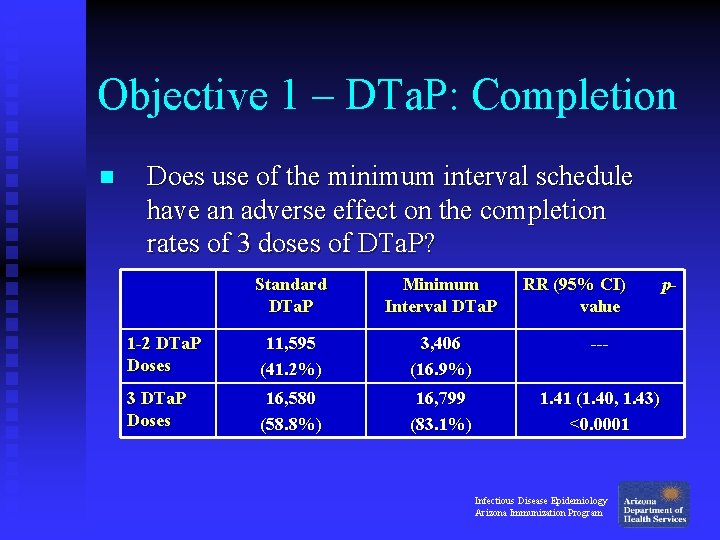
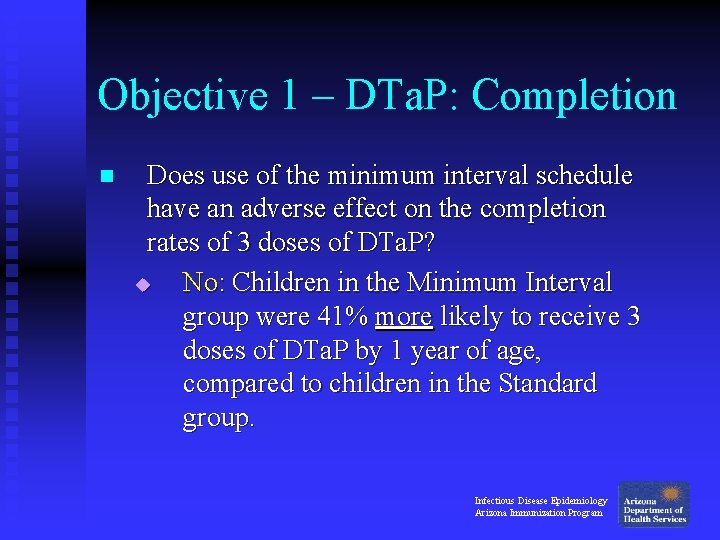
Objective 1 – DTa. P: Completion n Does use of the minimum interval schedule have an adverse effect on the completion rates of 3 doses of DTa. P? Standard DTa. P Minimum Interval DTa. P RR (95% CI) value 1 -2 DTa. P Doses 11, 595 (41. 2%) 3, 406 (16. 9%) --- 3 DTa. P Doses 16, 580 (58. 8%) 16, 799 (83. 1%) 1. 41 (1. 40, 1. 43) <0. 0001 Infectious Disease Epidemiology Arizona Immunization Program p-

Objective 1 – DTa. P: Completion n Does use of the minimum interval schedule have an adverse effect on the completion rates of 3 doses of DTa. P? u No: Children in the Minimum Interval group were 41% more likely to receive 3 doses of DTa. P by 1 year of age, compared to children in the Standard group. Infectious Disease Epidemiology Arizona Immunization Program

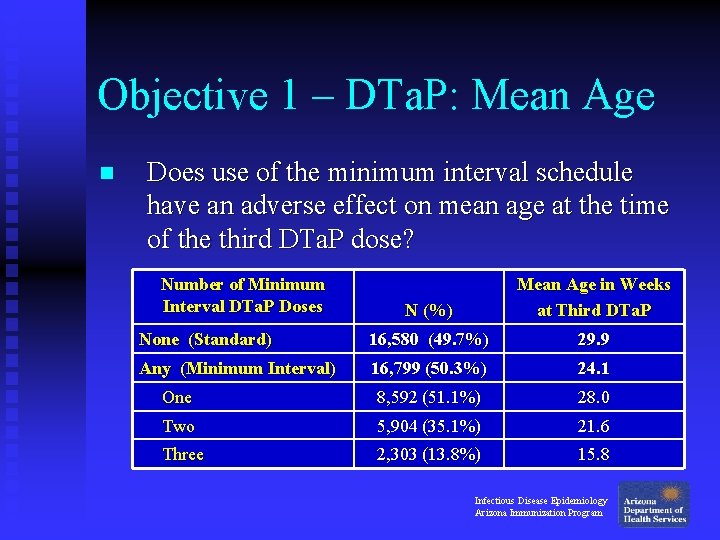
Objective 1 – DTa. P: Mean Age n Does use of the minimum interval schedule have an adverse effect on mean age at the time of the third DTa. P dose? Number of Minimum Interval DTa. P Doses N (%) Mean Age in Weeks at Third DTa. P None (Standard) 16, 580 (49. 7%) 29. 9 Any (Minimum Interval) 16, 799 (50. 3%) 24. 1 One 8, 592 (51. 1%) 28. 0 Two 5, 904 (35. 1%) 21. 6 Three 2, 303 (13. 8%) 15. 8 Infectious Disease Epidemiology Arizona Immunization Program

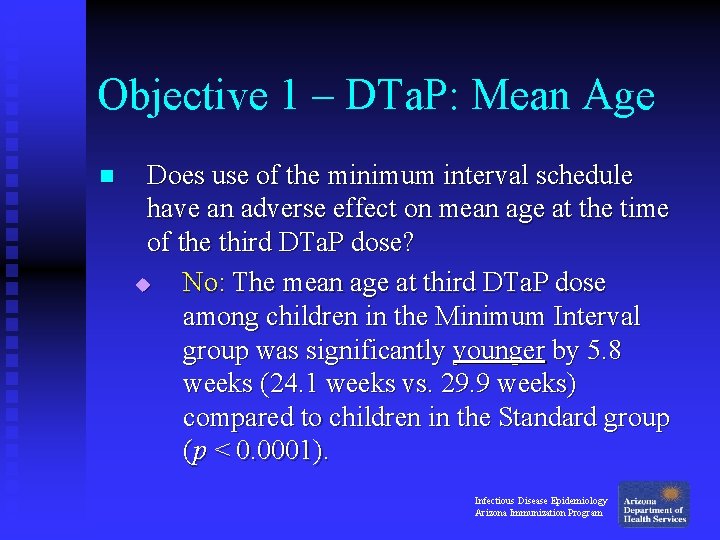
Objective 1 – DTa. P: Mean Age n Does use of the minimum interval schedule have an adverse effect on mean age at the time of the third DTa. P dose? u No: The mean age at third DTa. P dose among children in the Minimum Interval group was significantly younger by 5. 8 weeks (24. 1 weeks vs. 29. 9 weeks) compared to children in the Standard group (p < 0. 0001). Infectious Disease Epidemiology Arizona Immunization Program

Objective 2 – IPV n Compare the Standard group with the Minimum Interval group u Any IPV vaccinations received u Completion rates of 3 doses of IPV among those children receiving any IPV Infectious Disease Epidemiology Arizona Immunization Program

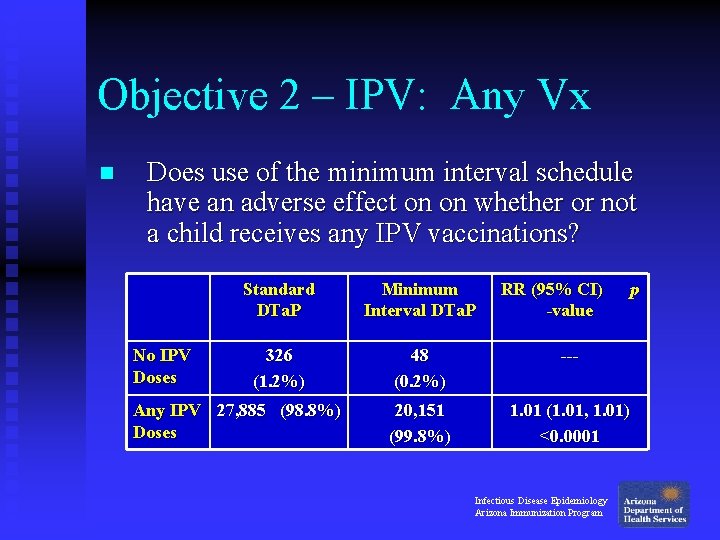
Objective 2 – IPV: Any Vx n Does use of the minimum interval schedule have an adverse effect on on whether or not a child receives any IPV vaccinations? No IPV Doses Standard DTa. P Minimum Interval DTa. P 326 (1. 2%) 48 (0. 2%) --- 20, 151 (99. 8%) 1. 01 (1. 01, 1. 01) <0. 0001 Any IPV 27, 885 (98. 8%) Doses RR (95% CI) -value Infectious Disease Epidemiology Arizona Immunization Program p

Objective 2 – IPV: Any Vx n Does use of the minimum interval schedule have an adverse effect on whether or not a child receives any IPV vaccinations? u No: Children in the Minimum Interval group were 1% more likely to receive any doses of IPV by 1 year of age, compared to children in the Standard group. Infectious Disease Epidemiology Arizona Immunization Program

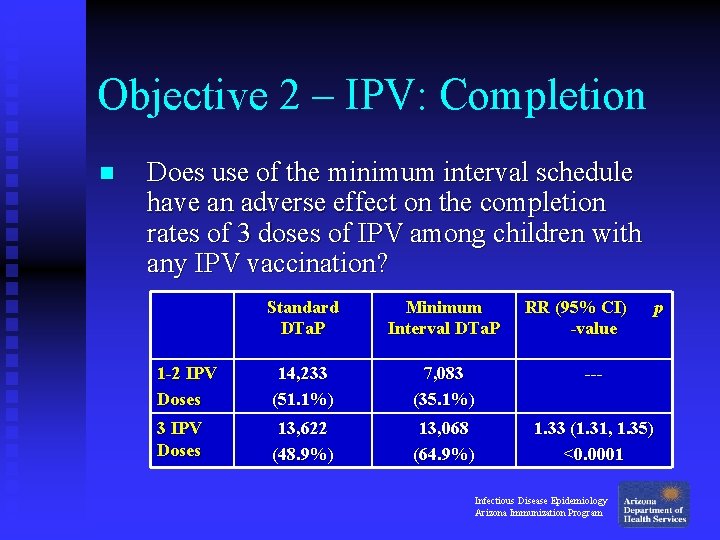
Objective 2 – IPV: Completion n Does use of the minimum interval schedule have an adverse effect on the completion rates of 3 doses of IPV among children with any IPV vaccination? Standard DTa. P Minimum Interval DTa. P RR (95% CI) -value 1 -2 IPV Doses 14, 233 (51. 1%) 7, 083 (35. 1%) --- 3 IPV Doses 13, 622 (48. 9%) 13, 068 (64. 9%) 1. 33 (1. 31, 1. 35) <0. 0001 Infectious Disease Epidemiology Arizona Immunization Program p

Objective 2 – IPV: Completion n Does use of the minimum interval schedule have an adverse effect on the completion rates of 3 doses of IPV among children with any IPV vaccination? u No: Children in the Minimum Interval group were 33% more likely to receive 3 doses of IPV by 1 year of age, compared to children in the Standard group. Infectious Disease Epidemiology Arizona Immunization Program

Objective 3 – PCV n Compare the Standard group with the Minimum Interval group u Any PCV vaccinations received u Completion rates of 3 doses of PCV among those children receiving any PCV Infectious Disease Epidemiology Arizona Immunization Program

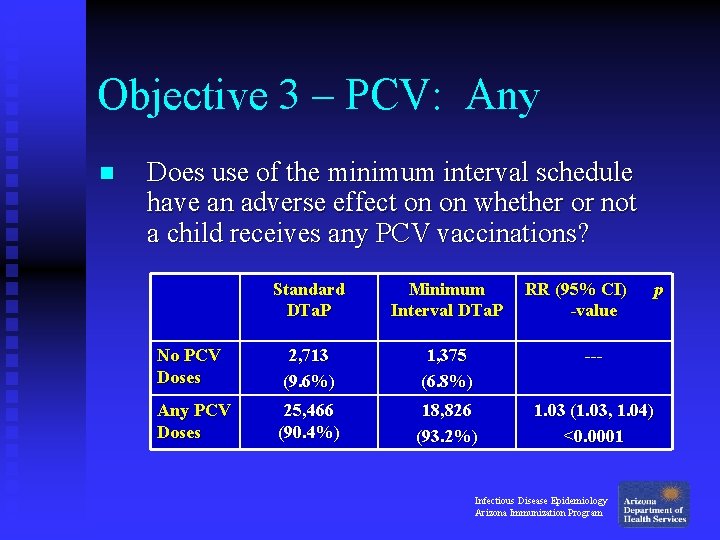
Objective 3 – PCV: Any n Does use of the minimum interval schedule have an adverse effect on on whether or not a child receives any PCV vaccinations? Standard DTa. P Minimum Interval DTa. P RR (95% CI) -value No PCV Doses 2, 713 (9. 6%) 1, 375 (6. 8%) --- Any PCV Doses 25, 466 (90. 4%) 18, 826 (93. 2%) 1. 03 (1. 03, 1. 04) <0. 0001 Infectious Disease Epidemiology Arizona Immunization Program p

Objective 3 – PCV: Any n Is there a difference in whether or not a child receives any PCV vaccinations? u No: Children in the Minimum Interval group were 3% more likely to receive any doses of PCV by 1 year of age, compared to children in the Standard group. Infectious Disease Epidemiology Arizona Immunization Program

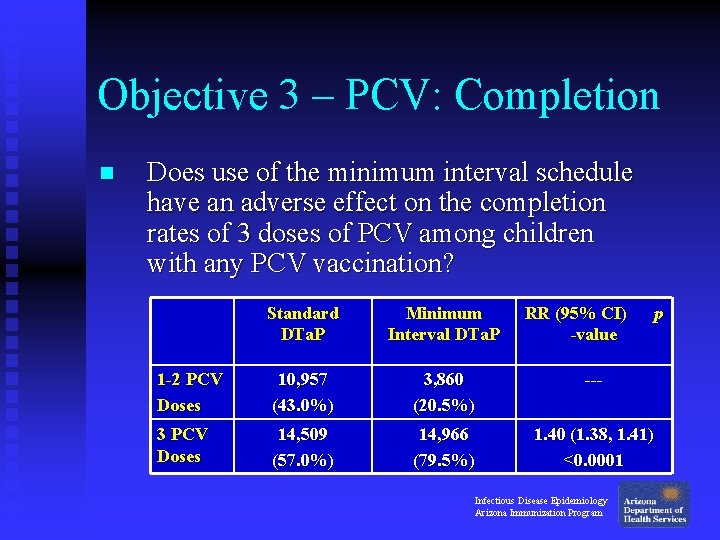
Objective 3 – PCV: Completion n Does use of the minimum interval schedule have an adverse effect on the completion rates of 3 doses of PCV among children with any PCV vaccination? Standard DTa. P Minimum Interval DTa. P RR (95% CI) -value 1 -2 PCV Doses 10, 957 (43. 0%) 3, 860 (20. 5%) --- 3 PCV Doses 14, 509 (57. 0%) 14, 966 (79. 5%) 1. 40 (1. 38, 1. 41) <0. 0001 Infectious Disease Epidemiology Arizona Immunization Program p

Objective 3 – PCV: Completion n Does use of the minimum interval schedule have an adverse effect on the completion rates of 3 doses of PCV among children with any PCV vaccination? u No: Children in the Minimum Interval group were 40% more likely to receive 3 doses of PCV by 1 year of age, compared to children in the Standard group. Infectious Disease Epidemiology Arizona Immunization Program

Limitations n n Underreporting of vaccinations to ASIIS u Estimated 13% underreporting u May have been larger in 2005 Does not address effect of minimum interval recommendations on morbidity Infectious Disease Epidemiology Arizona Immunization Program

Conclusions n During a state-wide pertussis outbreak: u It appears that recommending use of the minimum interval schedule for DTa. P does not have an adverse effect on the receipt of other childhood vaccines normally given at the same time. Infectious Disease Epidemiology Arizona Immunization Program

Conclusions n During their first year of life, children with any DTa. P vaccinations on the minimum interval schedule were: u 41% more likely to receive 3 doses of DTa. P (and to do so at a younger age), u 33% more likely to receive 3 doses of IPV, and u 40% more likely to receive 3 doses of PCV, compared to children on the standard interval DTa. P schedule. Infectious Disease Epidemiology Arizona Immunization Program

Future Research n n n Re-examine with two years of follow-up Compare children with standard DTa. P schedules from this population with children the year before the outbreak Determine if parents are bringing the children in for extra visits or if children are receiving the other vaccines on the same schedule as the minimum interval DTa. P doses. Infectious Disease Epidemiology Arizona Immunization Program

Acknowledgements n n n Arizona Immunization Program, Bureau of Epidemiology and Disease Control Services, Public Health Preparedness Services, Department of Health Services, State of Arizona t Michael Conklin Arizona Local Health Departments Rebecca Sunenshine, M. D. Infectious Disease Epidemiology Arizona Immunization Program