Effects of pressure change on chemical equilibrium Back

Effects of pressure change on chemical equilibrium

Back in Time: General gas law PV=n. RT <=> P= n. RT/V Pressure is directly proportional to number of moles and temperature, and is inversely proportional to the volume occupied by the gas
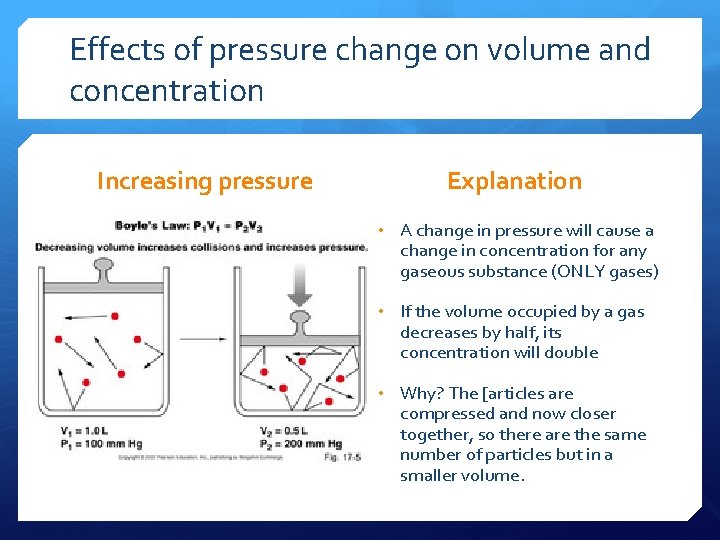
Effects of pressure change on volume and concentration Increasing pressure Explanation • A change in pressure will cause a change in concentration for any gaseous substance (ONLY gases) • If the volume occupied by a gas decreases by half, its concentration will double • Why? The [articles are compressed and now closer together, so there are the same number of particles but in a smaller volume.
Le Chatelier’s Principle applied to changes in pressure In a reaction system at equilibrium, any increase in pressure shifts the equilibrium in favor of a reaction producing the least number of gas molecules. Conversely, any decrease in pressure shifts the equilibrium in favor of a reaction producing the greatest number of gas molecules. Le Chatelier’s principle states that the reaction system reacts to the change in pressure in order to oppose this change. *Note: the system must have at least one substance in the gaseous state. Solids and liquids are incompressible, so a pressure change would not affect their concentration.
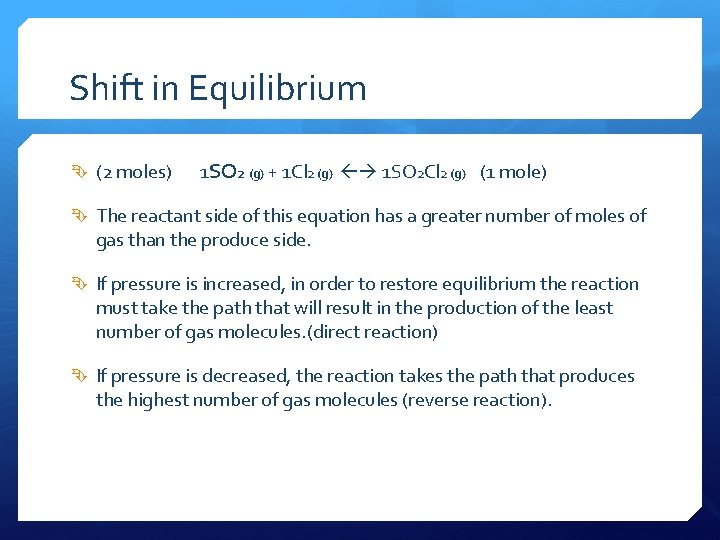
Shift in Equilibrium (2 moles) 1 SO 2 (g) + 1 Cl 2 (g) 1 SO 2 Cl 2 (g) (1 mole) The reactant side of this equation has a greater number of moles of gas than the produce side. If pressure is increased, in order to restore equilibrium the reaction must take the path that will result in the production of the least number of gas molecules. (direct reaction) If pressure is decreased, the reaction takes the path that produces the highest number of gas molecules (reverse reaction).
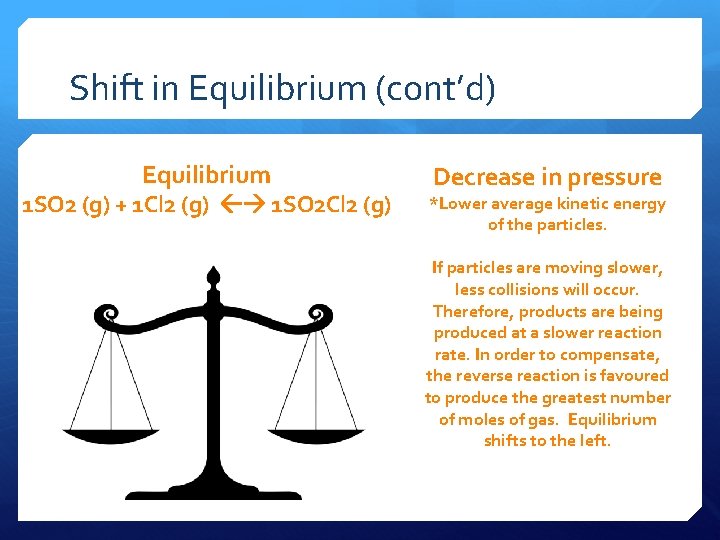
Shift in Equilibrium (cont’d) Equilibrium 1 SO 2 (g) + 1 Cl 2 (g) 1 SO 2 Cl 2 (g) Decrease in pressure *Lower average kinetic energy of the particles. If particles are moving slower, less collisions will occur. Therefore, products are being produced at a slower reaction rate. In order to compensate, the reverse reaction is favoured to produce the greatest number of moles of gas. Equilibrium shifts to the left.

1. System of N 2 O 4 (g) 2 NO 2 (g) at equilibrium At equilibrium, the presence of NO 2 gives the system a light brown color. NO 2 is dark brown, and is mixed with the colorless N 2 O 4, so it becomes light brown. Note: in the system, there are 2 moles of NO 2 for every 1 mole of N 2 O 4
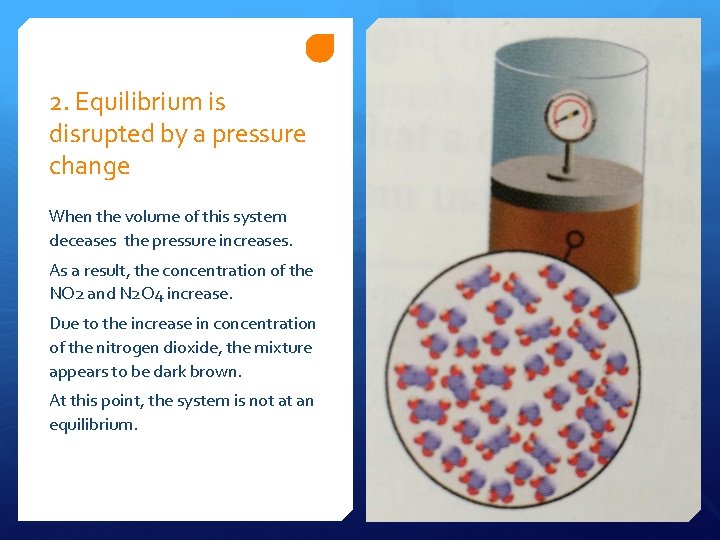
2. Equilibrium is disrupted by a pressure change When the volume of this system deceases the pressure increases. As a result, the concentration of the NO 2 and N 2 O 4 increase. Due to the increase in concentration of the nitrogen dioxide, the mixture appears to be dark brown. At this point, the system is not at an equilibrium.
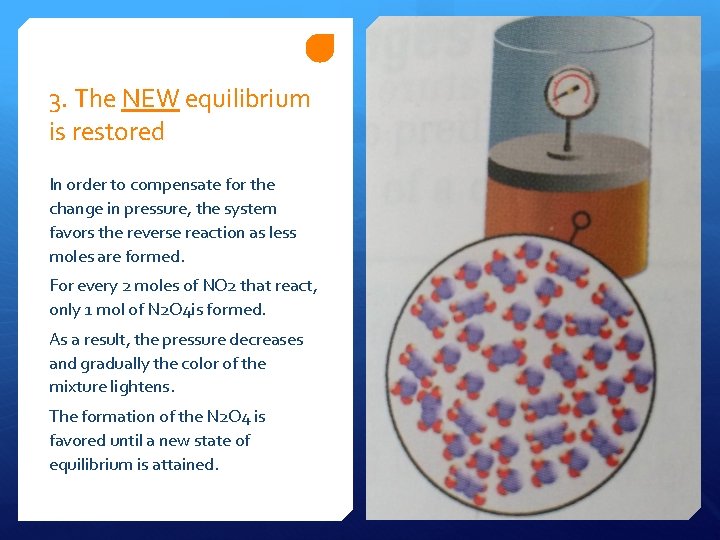
3. The NEW equilibrium is restored In order to compensate for the change in pressure, the system favors the reverse reaction as less moles are formed. For every 2 moles of NO 2 that react, only 1 mol of N 2 O 4 is formed. As a result, the pressure decreases and gradually the color of the mixture lightens. The formation of the N 2 O 4 is favored until a new state of equilibrium is attained.
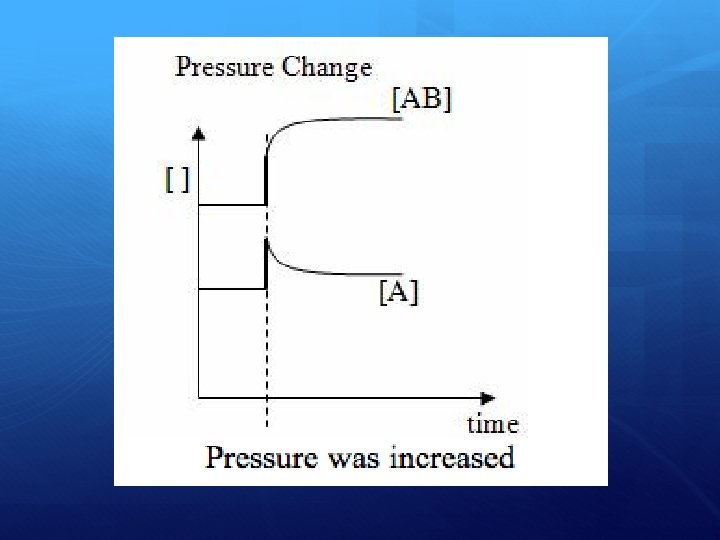

When reactants and products are equal in number of moles…. . A change in pressure of a system will not affect the reaction if there is an equal number of moles of reactants and products. Why? The same number of moles will be produced no matter which way the reaction occurs. As a result, equilibrium can’t move in any way that will reduce the pressure of the system.

Question Time!! What is the effect of an increase and decrease in pressure on the following reactions? : 1) SO 2(g) + Cl 2(g) SO 2 Cl 2 (g) 2) H 2 (g) + Cl 2 (g) 2 HCl (g)

Let’s see how well you did! 1) (2 moles of gas) SO 2 (g) + Cl 2 (g) SO 2 Cl 2 (g) (1 mole of gas) Increase in pressure: Equilibrium shifts to the right (favours direct reaction) since this is the path resulting in the least number of gas molecules. Decrease in pressure: Reverse reaction is favoured (equilibrium shifts to the left) since this is the path resulting in the greatest number of gas molecules. 2) (2 moles of gas) H 2 (g) + Cl 2 (g) 2 HCl (g) (2 moles of gas) An increase/decrease in pressure will have NO effect. No matter which path the reaction takes (direct or reverse), the same number of gas molecules will be produced.
- Slides: 13