Effective Sanitation Verification Elements Environment factors Continuum continuous












- Slides: 19

Effective Sanitation & Verification

Elements • Environment factors • Continuum / continuous improvement • Daily Sanitation: Back to basics – Sanitation GMP / 7 Steps. • Periodic Sanitation: Tear down & heat. • Verification of effective sanitation.

Environment • Staffing. – Turnover: • Maintaining training & documentation. – Thirds shift dynamics. • Necessity for flexible workforce (call-ins). • Room and Equipment. – HVAC (visibility during sanitation). – Accessibility (can you touch it / see it? ). – Keep it simple (can you disassemble without tools? ). • Continuum. – Sanitary design + Effective Sanitation + Traffic Patterns + GMPS + Dry/un-cracked flooring.

Lead to Ineffective Sanitation! • • • Aerosols. Spraying drains & drain components. Drains pooling/backing up. Hollow rollers, fixed sleeved assemblies, concave surfaces. Cross traffic. Lack of accessibility. Biofilms. Idle equipment (not being used). Standing moisture.

• • • High pressure water Compressed air Control buttons & screens Bearings Congestion in RTE area Cabinets in RTE areas Traffic patterns GMP requirements Sanitation process NOT documented/sequenced. How do you sustain protocols?

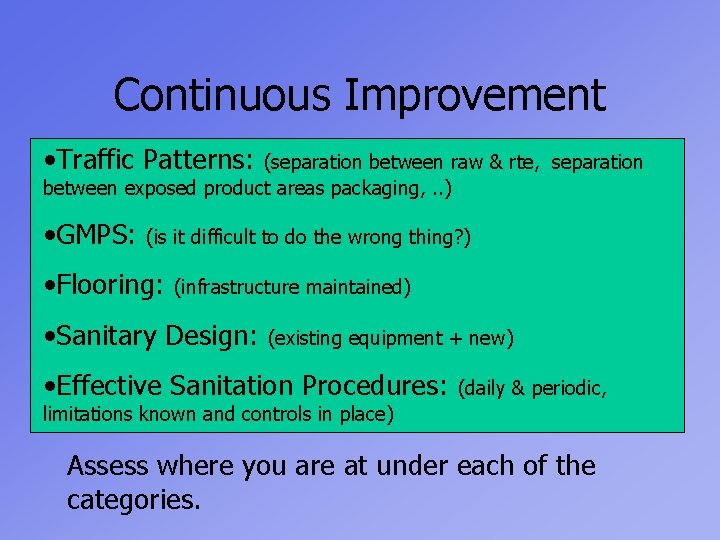
Continuous Improvement • Traffic Patterns: (separation between raw & rte, separation between exposed product areas packaging, . . ) • GMPS: (is it difficult to do the wrong thing? ) • Flooring: (infrastructure maintained) • Sanitary Design: (existing equipment + new) • Effective Sanitation Procedures: (daily & periodic, limitations known and controls in place) Assess where you are at under each of the categories.

Daily & Periodic Sanitation

Dry Clean 1 • Pre-sanitation task completed consistently (floors swept, equipment covered, materials removed, . . ). • Equipment disassembled to proper level to provide accessibility. • Dry clean completed Hollow member + cracked weld

Pre • Rinse until visually free of soils. Rinse 2 • Use lowest effective pressure to minimize aerosols and condensation. • Lower pressure reduces risk of cross contamination and machine damage. Multiple lap joints

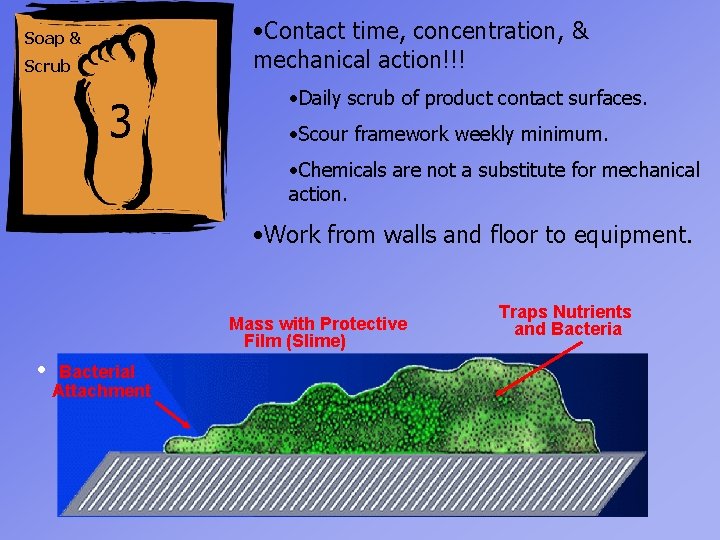
• Contact time, concentration, & mechanical action!!! Soap & Scrub 3 • Daily scrub of product contact surfaces. • Scour framework weekly minimum. • Chemicals are not a substitute for mechanical action. • Work from walls and floor to equipment. Mass with Protective Film (Slime) • Bacterial Attachment Traps Nutrients and Bacteria

• Order of applications (necessary to reduce cross contamination potential) Soap & Scrub 3 • Wall/floors then equipment • Avoid drying of chemicals • Mechanical action

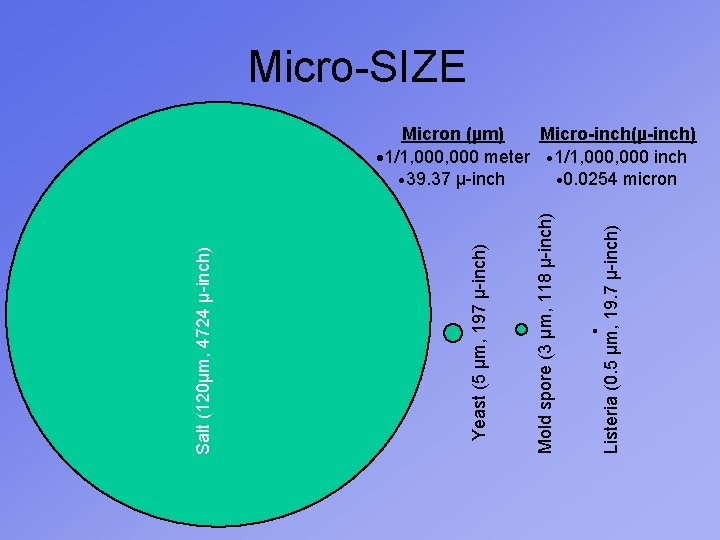
Listeria (0. 5 µm, 19. 7 µ-inch) Mold spore (3 µm, 118 µ-inch) Yeast (5 µm, 197 µ-inch) Salt (120µm, 4724 µ-inch) Micro-SIZE Micron (µm) Micro-inch(µ-inch) · 1/1, 000 meter · 1/1, 000 inch · 39. 37 µ-inch · 0. 0254 micron

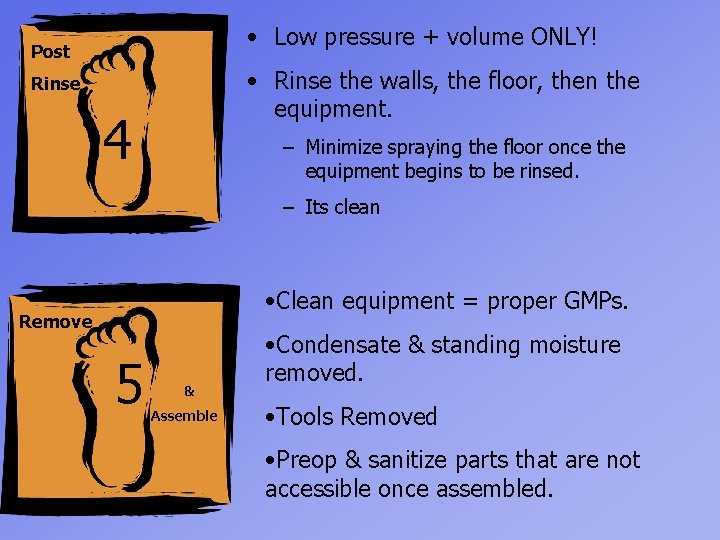
• Low pressure + volume ONLY! Post • Rinse the walls, the floor, then the equipment. Rinse 4 – Minimize spraying the floor once the equipment begins to be rinsed. – Its clean • Clean equipment = proper GMPs. Remove 5 & Assemble • Condensate & standing moisture removed. • Tools Removed • Preop & sanitize parts that are not accessible once assembled.

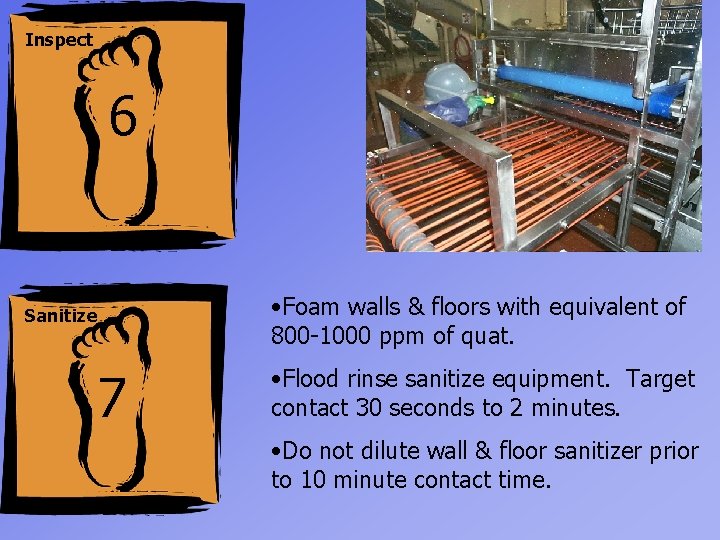
Inspect 6 Sanitize 7 • Foam walls & floors with equivalent of 800 -1000 ppm of quat. • Flood rinse sanitize equipment. Target contact 30 seconds to 2 minutes. • Do not dilute wall & floor sanitizer prior to 10 minute contact time.

Periodic Sanitation/ Tear Down for Access

Periodic Sanitation / Heat Steaming(165 F for 30 minutes) Dry heat (165 F for 4 hours)

Verification

Sanitation Effectiveness Verification Physical verification • Organoleptic (site, smell, taste) • Step 6 Microbiological Verification • Bioluminescence / ATP (immediate results) • Aerobic plate count (results in 2 -3 days) • Environmental Monitoring. (results in 3 -5 days)

Main Points 1. Continuous assessment & improvement. 2. Controls for sanitary design limitations. 3. Sanitation GMP: Providing mechanical action and limiting cross contamination opportunities. 4. Verify: Bioluminescence (go/no-go), Aerobic Plate Count (trend).