EFFECT OF SURFACTANTS ON SOLUBILITY OF POORLY SOLUBLE
EFFECT OF SURFACTANTS ON SOLUBILITY OF POORLY SOLUBLE DRUGS

CONTENTS Introduction BCS classification Solubility Surfactant mechanism and its uses Need of surfactants in solubility of poorly soluble drugs Solubility enhancement by following techniques Micro emulsions Micellar solubilisation Sedds Recent developments and advances through surfactants in drugs • Conclusion • • •
Introduction • Recently synthesized drug that are being discovered are lipophillic in nature and have poor aqueos solubility, there by possing problem in their solubility. • Because of their low aqueous solubility and low permeability, dissolution and/or release rate from delivery system rate –limiting step in their absorption and systemic bioavailability. • More than 60% of potential drug products suffer from poor water solubility. • For therapeutic delivery of lipophillic active moieties (BCS class II drugs), • lipid based formulations are inviting increasing attention. • Currently a number of technologies are available to deal with the poor solubility of drugs.
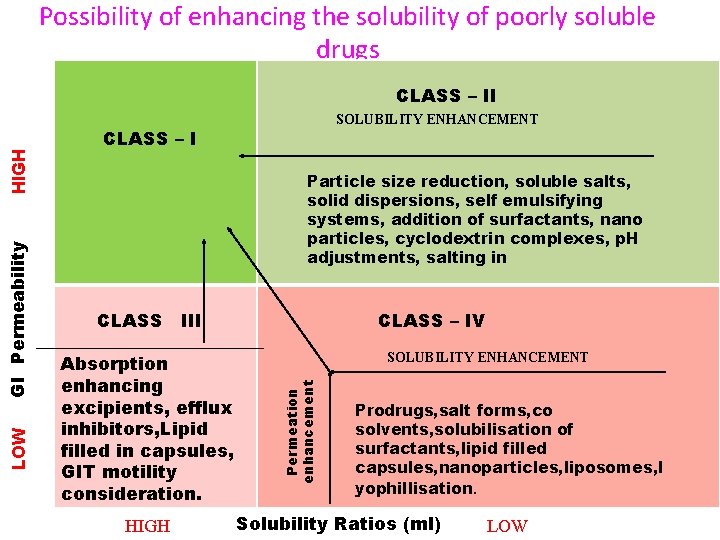
Possibility of enhancing the solubility of poorly soluble drugs SOLUBILITY ENHANCEMENT CLASS – I Particle size reduction, soluble salts, solid dispersions, self emulsifying systems, addition of surfactants, nano particles, cyclodextrin complexes, p. H adjustments, salting in CLASS III Absorption enhancing excipients, efflux inhibitors, Lipid filled in capsules, GIT motility consideration. HIGH CLASS – IV SOLUBILITY ENHANCEMENT Permeation enhancement LOW GI Permeability HIGH CLASS – II Prodrugs, salt forms, co solvents, solubilisation of surfactants, lipid filled capsules, nanoparticles, liposomes, l yophillisation. Solubility Ratios (ml) LOW

solubility • Solubility is the phenomenon of dissolution of solid in liquid phase to give a homogenous system. • Its measured in kilogram/mcube.
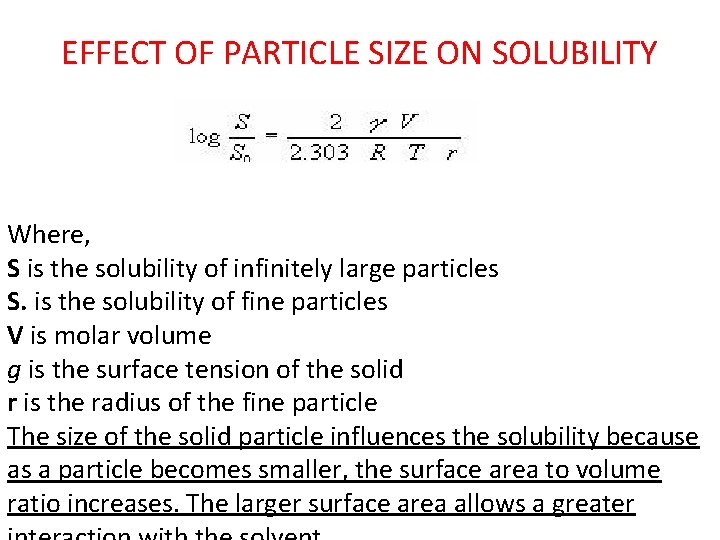
EFFECT OF PARTICLE SIZE ON SOLUBILITY Where, S is the solubility of infinitely large particles S. is the solubility of fine particles V is molar volume g is the surface tension of the solid r is the radius of the fine particle The size of the solid particle influences the solubility because as a particle becomes smaller, the surface area to volume ratio increases. The larger surface area allows a greater

Role of surfactants • To achieve the sink condition in dissolution medium by addition of cationic, anionic, nonionic surfactants. • Surfactants form micelles which improves the solubility of poorly soluble drugs. • They reduce the interfacial tension between two layers.
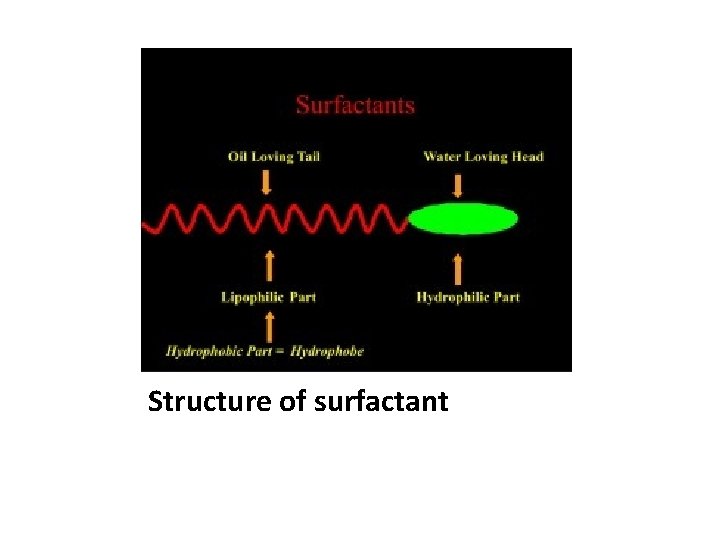
Structure of surfactant
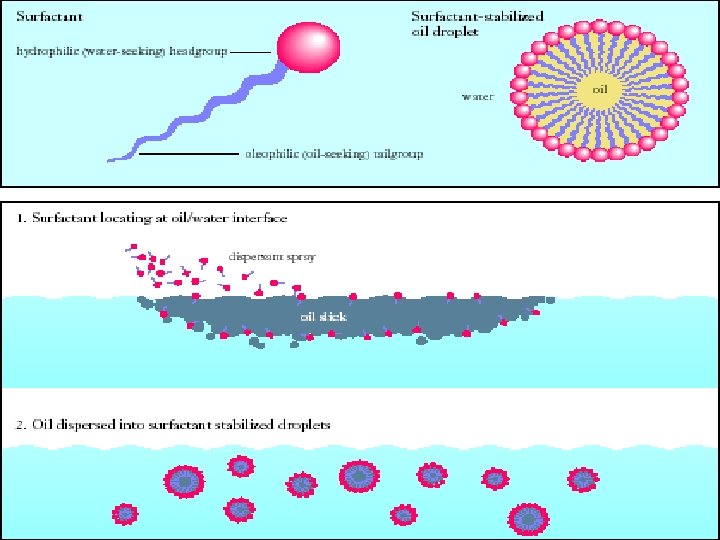
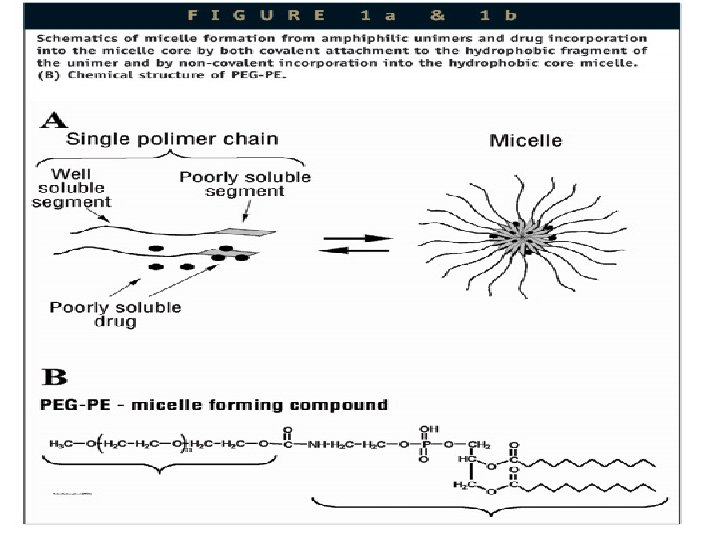
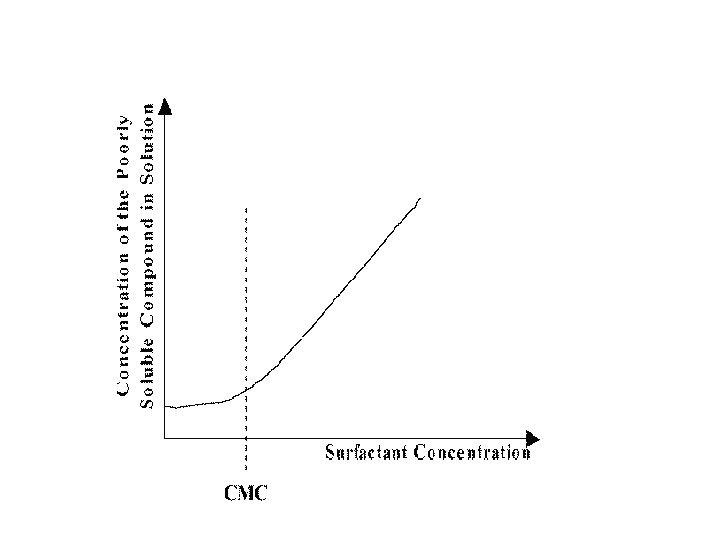
Surfactants increase solubility through following techniques • • Microemulsion formation Micellar solubilisation Self emulsifying drug delivery system Nanosuspensions
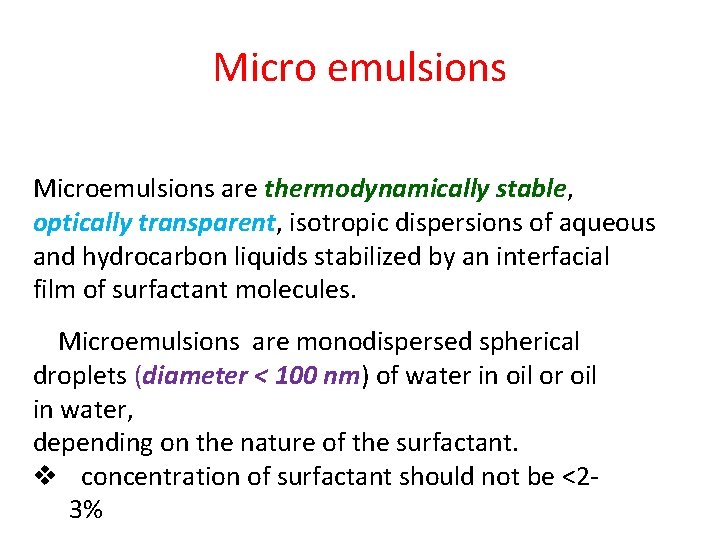
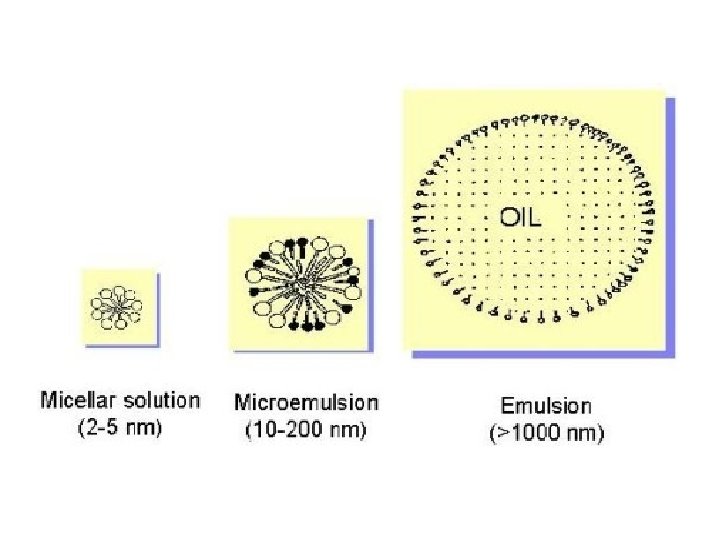
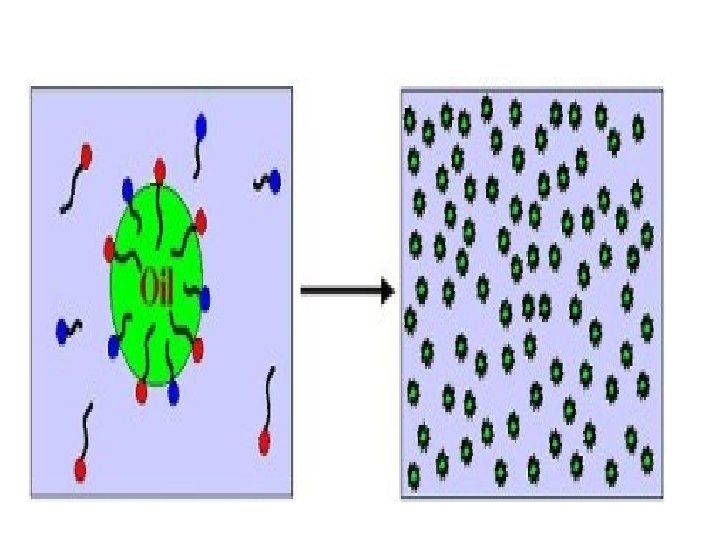
Micro emulsions Microemulsions are thermodynamically stable, optically transparent, isotropic dispersions of aqueous and hydrocarbon liquids stabilized by an interfacial film of surfactant molecules. Microemulsions are monodispersed spherical droplets (diameter < 100 nm) of water in oil or oil in water, depending on the nature of the surfactant. v concentration of surfactant should not be <2‐ 3%
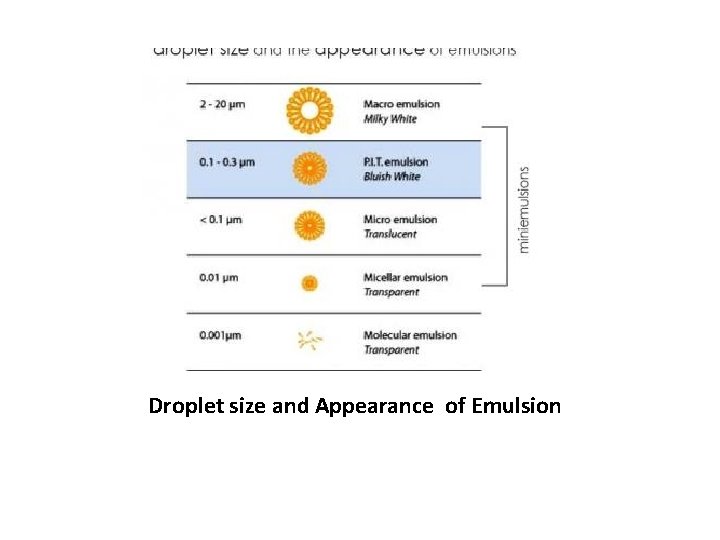
Droplet size and Appearance of Emulsion
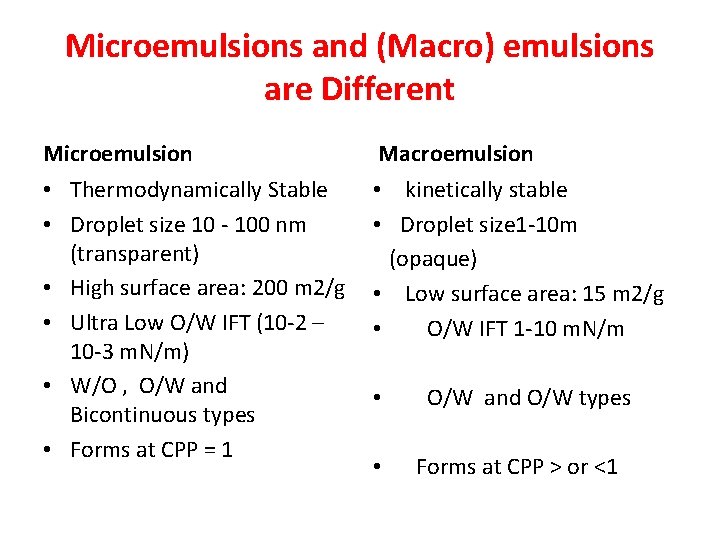
Microemulsions and (Macro) emulsions are Different Microemulsion Macroemulsion • Thermodynamically Stable • Droplet size 10 ‐ 100 nm (transparent) • High surface area: 200 m 2/g • Ultra Low O/W IFT (10‐ 2 – 10‐ 3 m. N/m) • W/O , O/W and Bicontinuous types • Forms at CPP = 1 • kinetically stable • Droplet size 1‐ 10 m (opaque) • Low surface area: 15 m 2/g • O/W IFT 1‐ 10 m. N/m • • O/W and O/W types Forms at CPP > or <1
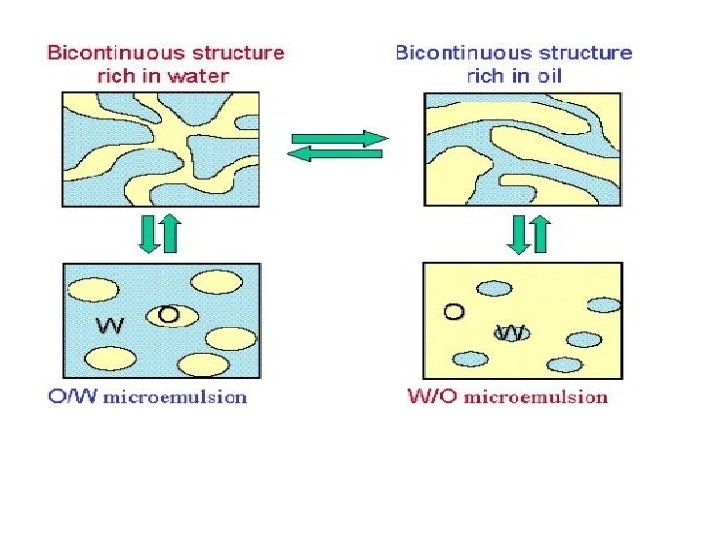
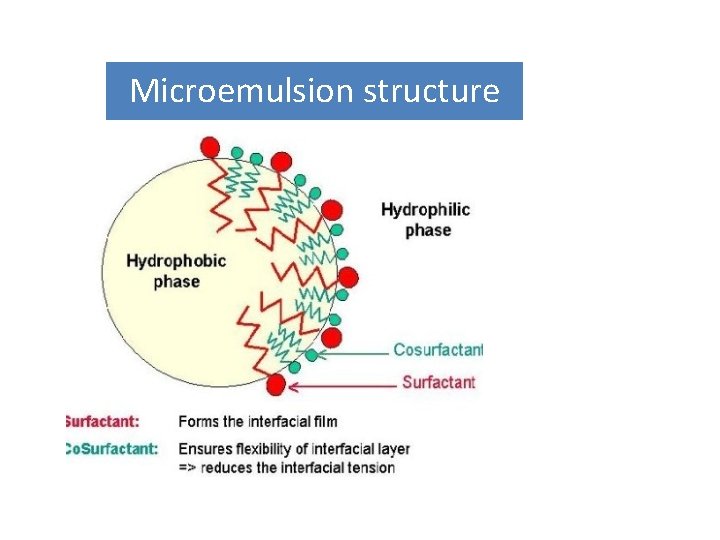
Microemulsion structure
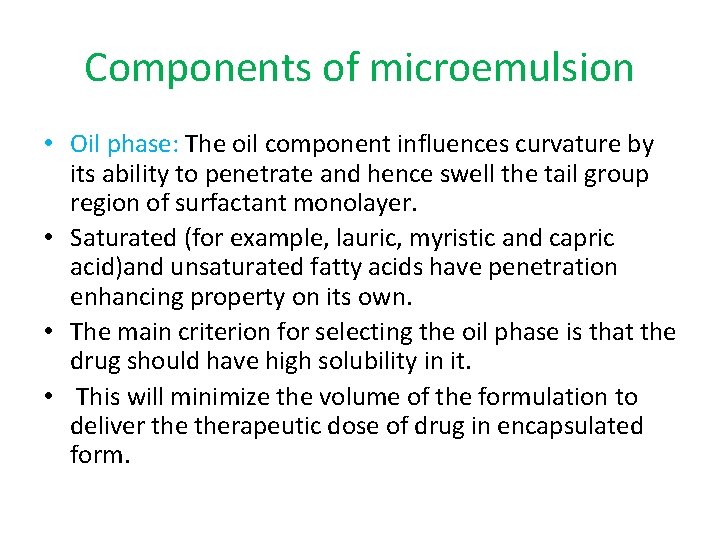
Components of microemulsion • Oil phase: The oil component influences curvature by its ability to penetrate and hence swell the tail group region of surfactant monolayer. • Saturated (for example, lauric, myristic and capric acid)and unsaturated fatty acids have penetration enhancing property on its own. • The main criterion for selecting the oil phase is that the drug should have high solubility in it. • This will minimize the volume of the formulation to deliver therapeutic dose of drug in encapsulated form.
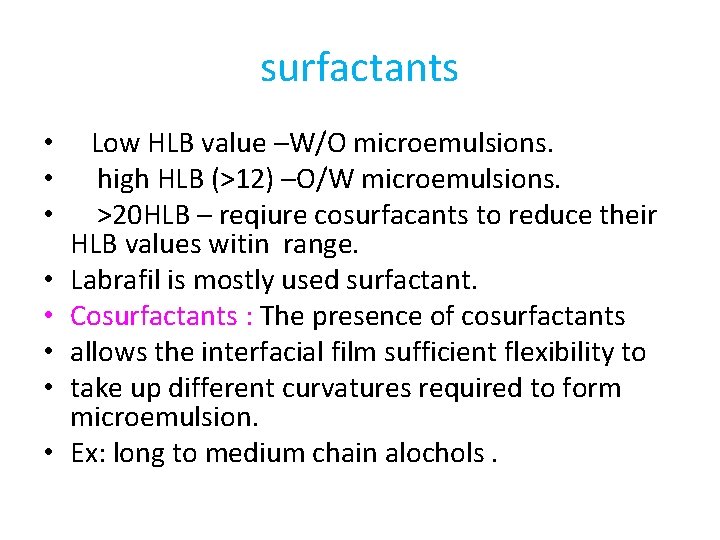
surfactants • • Low HLB value –W/O microemulsions. high HLB (>12) –O/W microemulsions. >20 HLB – reqiure cosurfacants to reduce their HLB values witin range. Labrafil is mostly used surfactant. Cosurfactants : The presence of cosurfactants allows the interfacial film sufficient flexibility to take up different curvatures required to form microemulsion. Ex: long to medium chain alochols.
Theories Of Micro-emulsion Formation • Historically, three approaches have been used to explain micro‐emulsion formation and stability. These are: • (i)Interfacial or mixed film theories • (ii)Solubilization theories • (iii)Thermodynamic treatments

METHODS OF PREPARATION • PHASE TITRATION METHOD • PHASE INVERSION METHOD • PHASE TITRATION METHOD: Microemulsions are prepared by the spontaneous emulsification method (phase titration method) and can be depicted with help of phase diagrams •
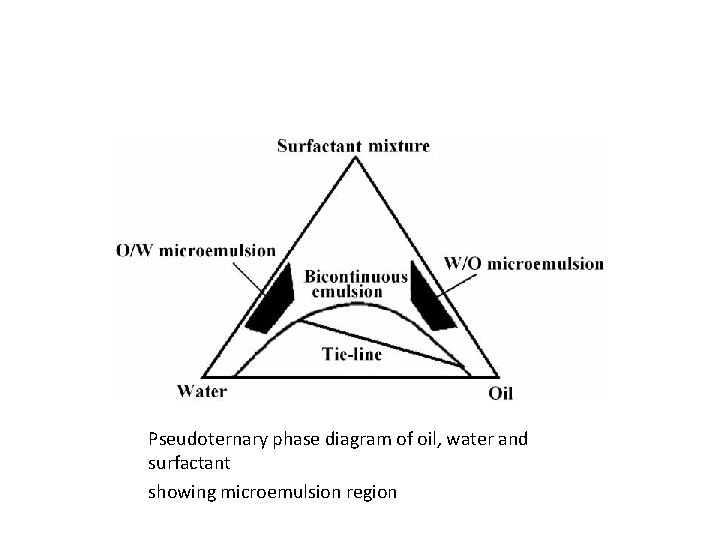
Pseudoternary phase diagram of oil, water and surfactant showing microemulsion region
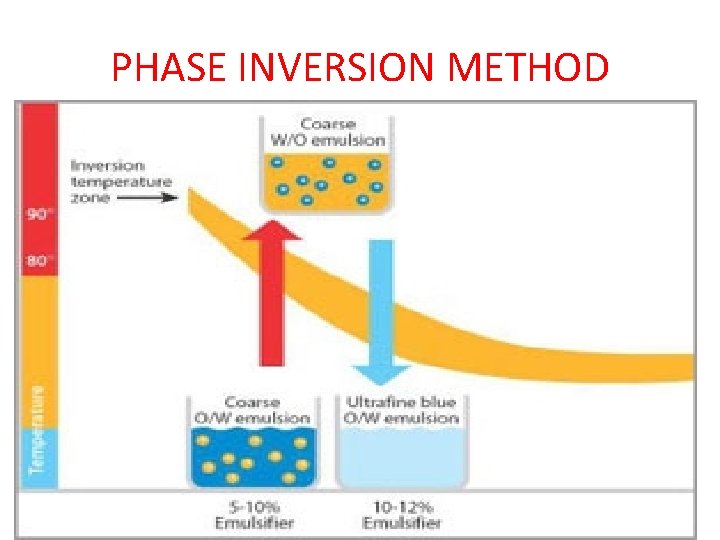
PHASE INVERSION METHOD
• Phase inversion temperature emulsions, known as P. I. T. emulsions, are fine, stable emulsions which can be thinned with water as required. • They are produced through a process which makes intelligent use of a natural property of oil‐in‐water emulsions. • The consistency and structure of oil/water mixtures depend in part on temperature. To produce P. I. T. emulsions, the oil‐in‐water mixture is first slowly heated. • At a temperature of 81 to 89 degrees Celsius, a fine emulsion is formed. When heated further, it is transformed into a water‐in‐oil emulsion. • Subsequent rapid cooling to about 30 degrees stabilizes the fine P. I. T. emulsion in the inversion phase. • o/w – w/o The consistency and structure of oil-in-water emulsions are primarily dependent on temperature. There is a range (81 -89°C) in which P. I. T. emulsions with particularly small oil droplets occur without the need for any major intervention

ADVANTAGES • • • Thermodynamically stable. Require minimum energy formation. Improved drug solubility and bioavailability. Drug targeting and controlled release. Microemulsions formation is reversible. Improves the efficacy of a drug. • Allows the reduced dose and side effects.
DISADVATNAGES • • • Large concentration of surfactant and co‐surfactant necessary for stabilizing the nanodroplets. Limited solubilizing capacity for high‐melting substances. The surfactant must be nontoxic for using pharmaceutical applications. Micro emulsion stability is influenced by environmental parameters such as temperature and p. H. These parameters change upon micro emulsion delivery to patients.
• Micro-emulsion products: Examples of poorly soluble compounds that use micro‐emulsion • are the HIV protease inhibitor tipranavir (Aptivus capsules, boehringer ) • The category defining immunosuppressant cyclosporin A, USP modified (Neoral capsules, Novartis AG)
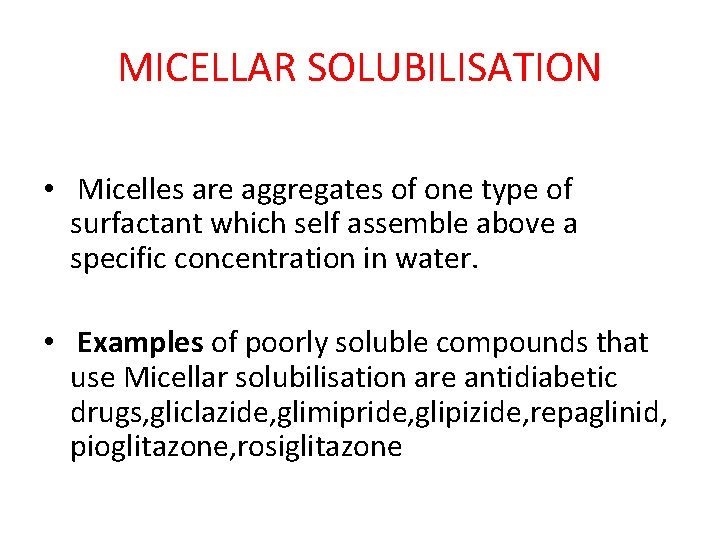
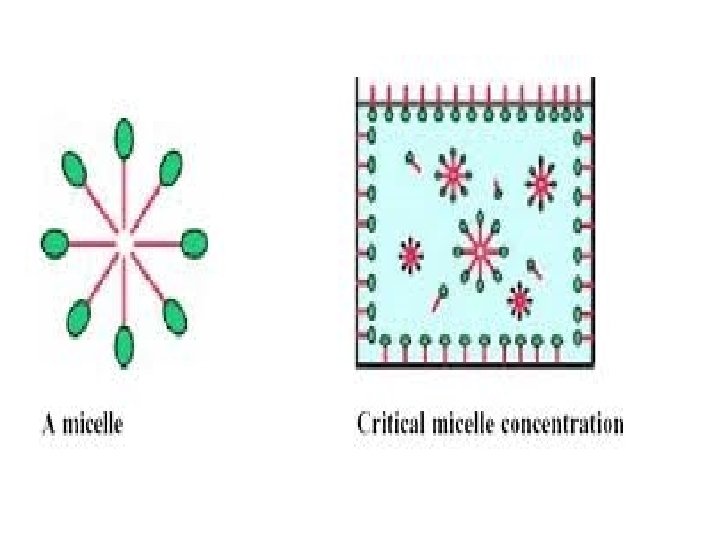
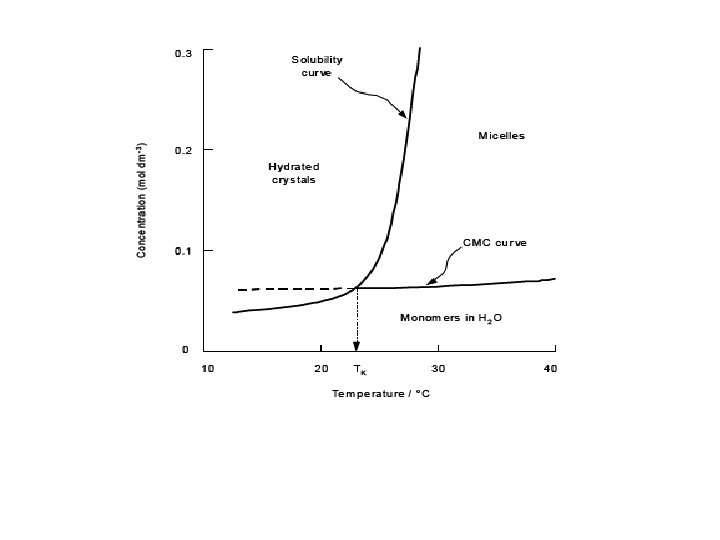
MICELLAR SOLUBILISATION • Micelles are aggregates of one type of surfactant which self assemble above a specific concentration in water. • Examples of poorly soluble compounds that use Micellar solubilisation are antidiabetic drugs, gliclazide, glimipride, glipizide, repaglinid, pioglitazone, rosiglitazone
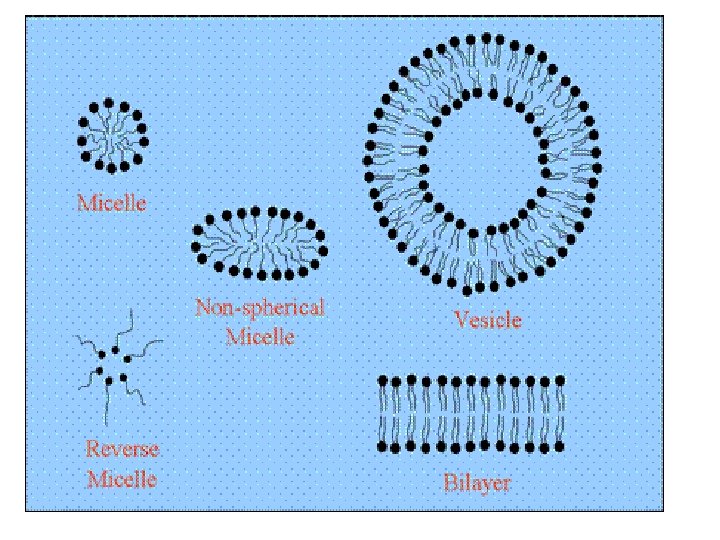
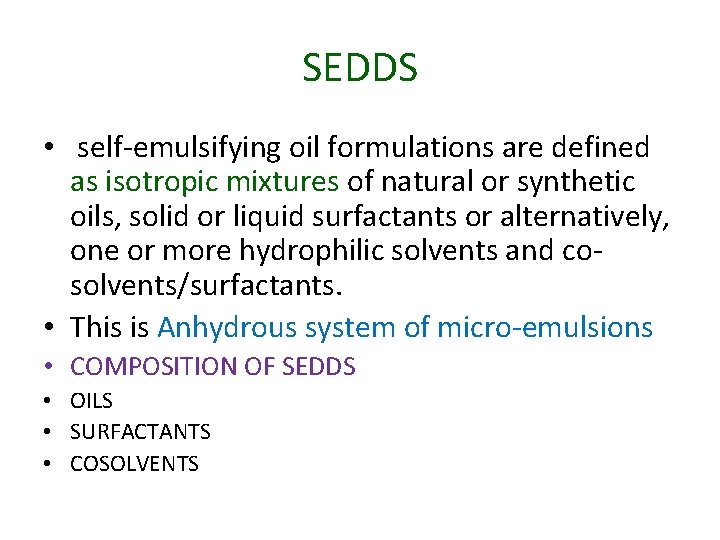
SEDDS • self‐emulsifying oil formulations are defined as isotropic mixtures of natural or synthetic oils, solid or liquid surfactants or alternatively, one or more hydrophilic solvents and co‐ solvents/surfactants. • This is Anhydrous system of micro‐emulsions • COMPOSITION OF SEDDS • OILS • SURFACTANTS • COSOLVENTS
FORMULATION OF SEDDS • Drug mixture+oil+surfactant+cosolvent=sedds • Optimal oil –surfactant ratio is required. • So, the design of an optimal SEDDS requires preformulation solubility and phase diagram studies. • In case of prolonged sedds gelling agent is required. •
Characterization of Self‐Emulsifying Drug Delivery Systems • • • Visual assessment Turbidity measurement Droplet size Zeta potential measurement Determination of emulsification time

Technique of Solid Self. Emulsifying Drug Delivery System Development • • Solid carriers Spray drying Melt extrusion Dry emulsion
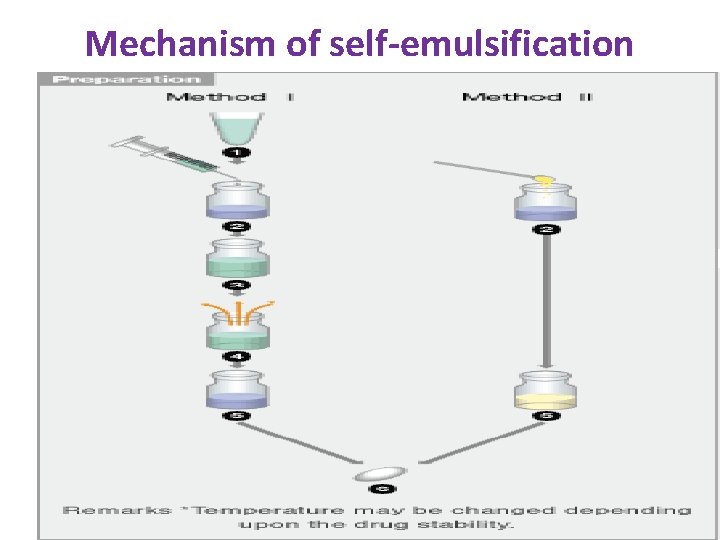
Mechanism of self-emulsification
 drug is dissolved in suitable solvent +solubilizer‐‐‐heat to 50 c [ Method I ](1) drug is dissolved in suitable solvent +solubilizer‐‐‐heat to 50 c](http://slidetodoc.com/presentation_image_h2/ea802dd6f58aeef025a4bef0a30a3b6b/image-38.jpg)
[ Method I ](1) drug is dissolved in suitable solvent +solubilizer‐‐‐heat to 50 c for 1 hr—concentrated solution of drug is formed‐‐‐moulded into soft capsules. . [ Method II ](2) Depending on the drug, it can be dissolved I solubiliser and followed same procedure of method 1
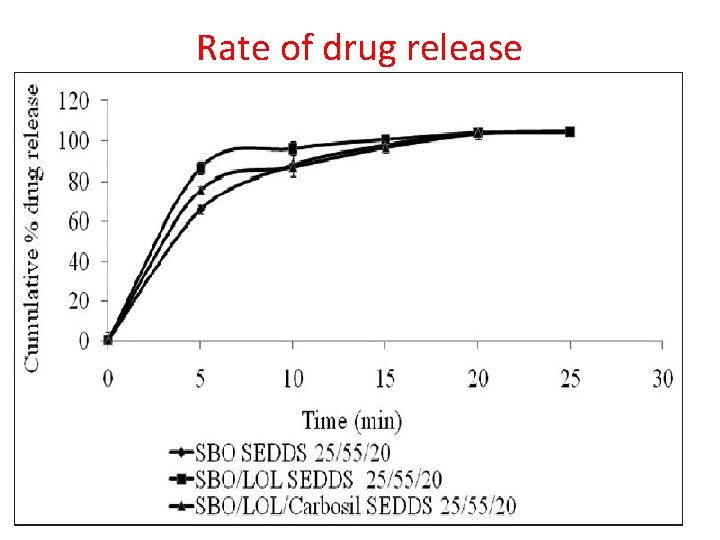
Rate of drug release
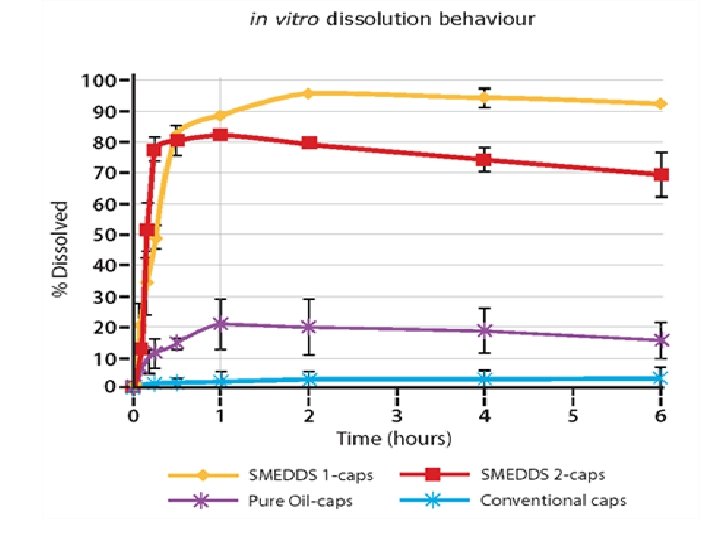
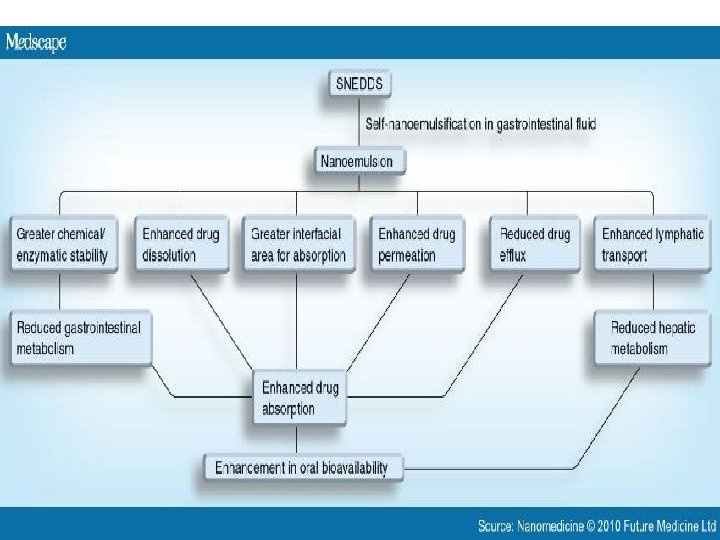
Applications • Improvement in Solubility and bioavailabilityketoprofen • distribution, and increase the dissolution and permeability. • SEDDSs protect drugs against hydrloysis by enzymes in GI tract. • Reduce hepatic first –pass metabolism. • Protection against Biodegradation

ADVANTAGES • • • 1. Enhanced oral bioavailability enabling reduction in dose. 2. More consistent temporal profiles of drug absorption. 3. Selective targeting of drug(s) toward specific. absorption window in GIT. 4. Protection of drug(s) from the hostile environment in gut 5. Control of delivery profiles. 6. Reduced variability including food effects. 7. Protective of sensitive drug substances. 8. High drug payloads. 9. Liquid or solid dosage forms.
Disadvantages • assessment of the formulations is difficult. • Traditional dissolution methods do not work, because these formulations depends on digestion prior to release drug. • in vitro model needs further development and validation before its strength is evaluated. • Futher development based on invitro‐in vivo studies.
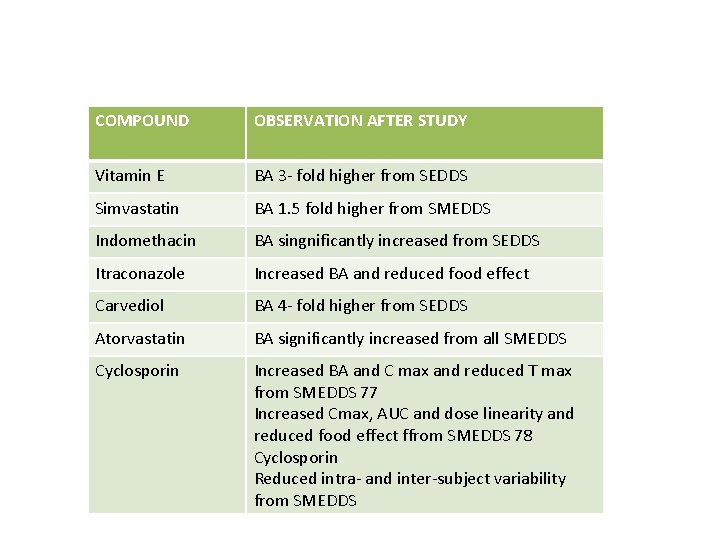
COMPOUND OBSERVATION AFTER STUDY Vitamin E BA 3‐ fold higher from SEDDS Simvastatin BA 1. 5 fold higher from SMEDDS Indomethacin BA singnificantly increased from SEDDS Itraconazole Increased BA and reduced food effect Carvediol BA 4‐ fold higher from SEDDS Atorvastatin BA significantly increased from all SMEDDS Cyclosporin Increased BA and C max and reduced T max from SMEDDS 77 Increased Cmax, AUC and dose linearity and reduced food effect ffrom SMEDDS 78 Cyclosporin Reduced intra‐ and inter‐subject variability from SMEDDS
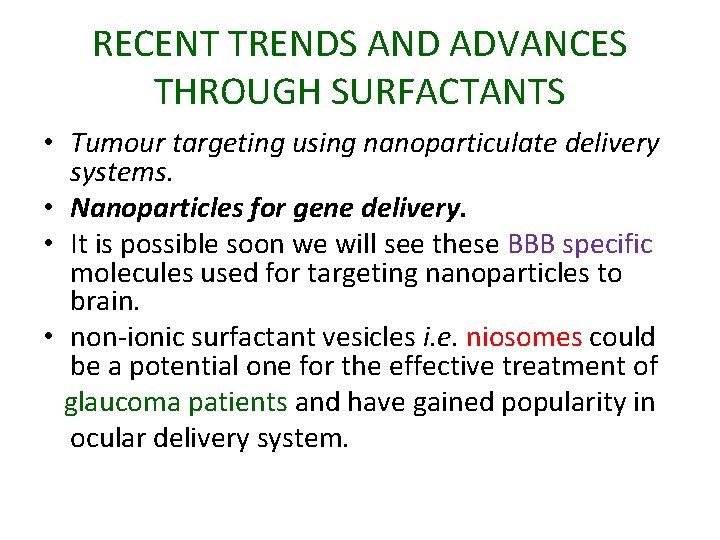
RECENT TRENDS AND ADVANCES THROUGH SURFACTANTS • Tumour targeting using nanoparticulate delivery systems. • Nanoparticles for gene delivery. • It is possible soon we will see these BBB specific molecules used for targeting nanoparticles to brain. • non‐ionic surfactant vesicles i. e. niosomes could be a potential one for the effective treatment of glaucoma patients and have gained popularity in ocular delivery system.

• Pharmaceutical dosage forms --enhancing metered dose inhalers, nebulisars, like salbutamol, ipratropium bromide. • Self emulsifying drug delivery system improved the solubility and bioavailability of many poorly soluble drugs. • surfactants are used to cure disease like Infant respiratory distress syndrome. • Microemulsions brought in success the , and improved the solubility of poorly soluble drugs like cyclosporine, paclitaxel. • Furthermore, they can be employed for challenging tasks such as carrying chemotherapeutic agents to neoplastic cells.

Conclusion • Surfactants improve the solubility of poorly soluble drugs by • Improving the dissolution rates • Reducing the interfacial tension • Formation of micelles • Formation of micro‐emulsions • And by SEDDS • And there by now it is possible to improve the solubility of poorly soluble drugs.

Biliography • Danielsson, I. ; Lindman, B. Colloids Surf. A 1981, 3, 391. • 2. C. Leuner, J. Dressman, Improving drug solubility for oral delivery using solid dispersions, Eur. J. Pharm. Biopharm. ; 2000; 50; 47– 60. • A. T. Florence solubilisation by surface active agents 1968 • Solubilisation techinques of drugs by yalkwosky 1: 15 • . A. T. M. Serajuddin, P. C. Sheen, M. A. Augustine, Improved dissolution of poorly water‐soluble drug from solid dispersions in polyethylene: Polysorbate 80 mixture, J. Pharm. Sci. ; 1990; 77; 463– 464.

• Physical Pharmacy, Alfred Martin, 4 th Edition, Page No: 541: 542. • Hoar T. P. and Schulman J. H. “Transparent water in oil dispersions: the oleopathic hydromicelle”. Nature 152: 102 – 103, 1943. • Attwood D. Microemulsions in Colloidal drug delivery systems (J. Kreuter ed. ), Marcel Dekker, New York 1994. • Kreilgaard M. “Influence of microemulsions on cutaneous drug delivery”. in Bulletin

Thank you







- Slides: 59