Effect of inhibitor on enzyme activity Inhibitors decrease
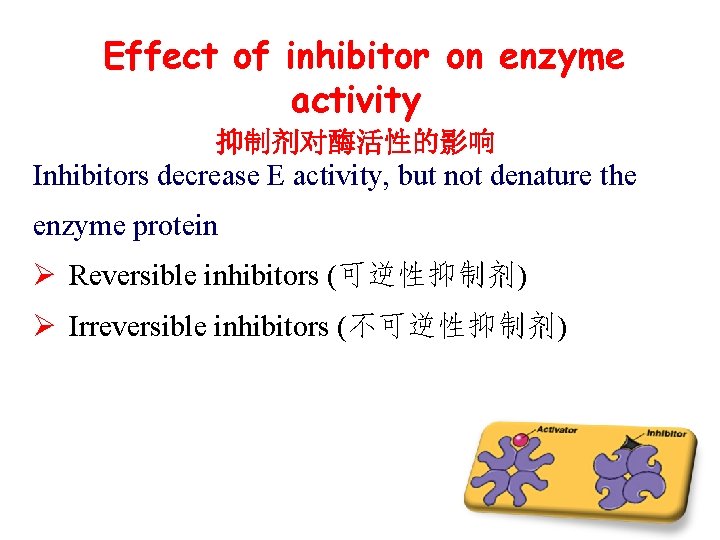
Effect of inhibitor on enzyme activity 抑制剂对酶活性的影响 Inhibitors decrease E activity, but not denature the enzyme protein Ø Reversible inhibitors (可逆性抑制剂) Ø Irreversible inhibitors (不可逆性抑制剂)

Irreversible inhibitors 不可逆抑制剂 Ø The inhibitor forms covalent or very strong noncovalent bonds with the essential groups in active center of an enzyme Ø Can not be removed by dialysis(透析), but can be removed by a special reagent
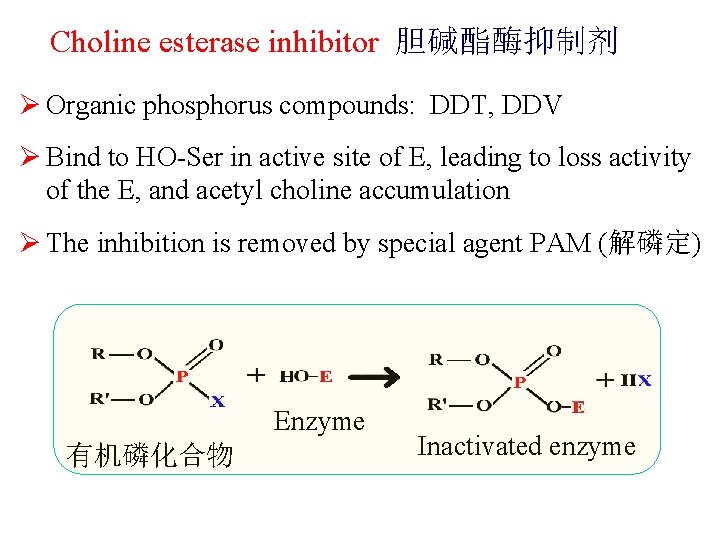
Choline esterase inhibitor 胆碱酯酶抑制剂 Ø Organic phosphorus compounds: DDT, DDV Ø Bind to HO-Ser in active site of E, leading to loss activity of the E, and acetyl choline accumulation Ø The inhibition is removed by special agent PAM (解磷定) Enzyme 有机磷化合物 Inactivated enzyme
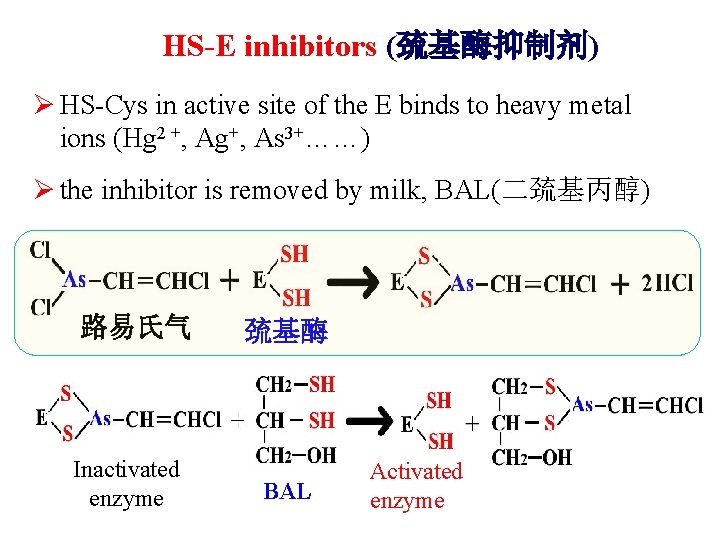
HS-E inhibitors (巯基酶抑制剂) Ø HS-Cys in active site of the E binds to heavy metal ions (Hg 2 +, Ag+, As 3+……) Ø the inhibitor is removed by milk, BAL(二巯基丙醇) 路易氏气 Inactivated enzyme 巯基酶 BAL Activated enzyme

Reversible inhibitors 可逆性抑制剂** Ø The inhibitor forms weak, non-covalently bonds that readily dissociate from E protein, and decreases the E activity --competitive inhibitor (竞争性抑制剂) --noncompetitive inhibitor (非竞争性抑制剂) --uncompetitive inhibitor (反竞争性抑制剂)
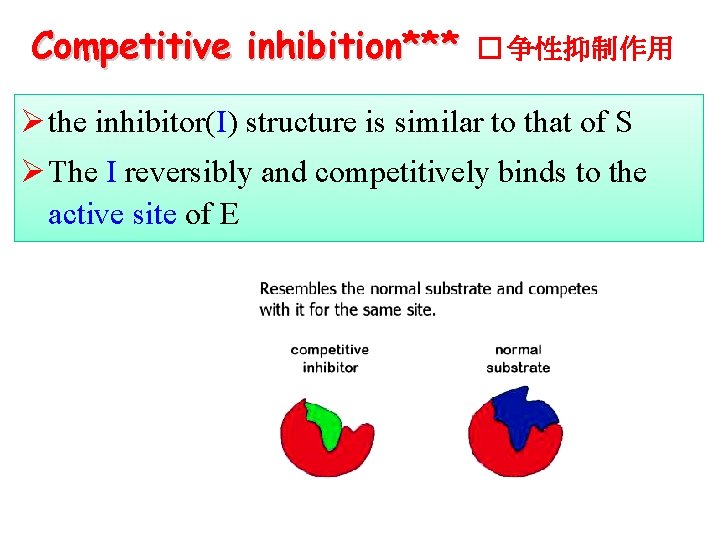
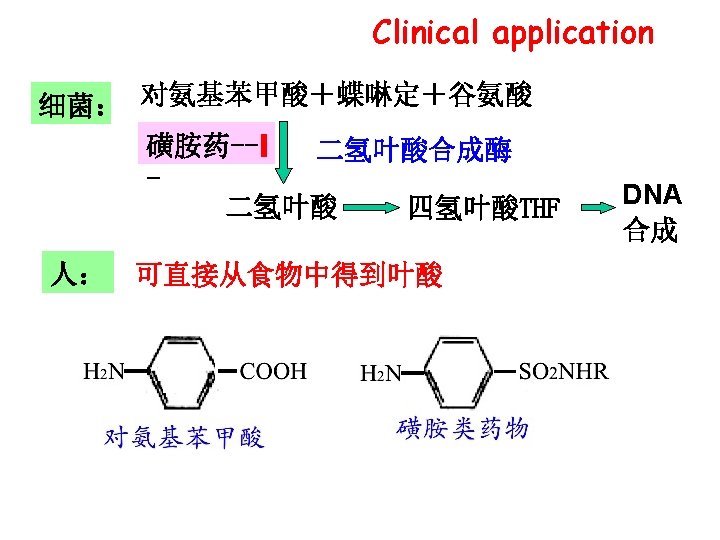
Competitive inhibition*** � 争性抑制作用 Ø the inhibitor(I) structure is similar to that of S Ø The I reversibly and competitively binds to the active site of E
![Competitive inhibition [S]↑ inhibition↓ Competitive inhibition [S]↑ inhibition↓](http://slidetodoc.com/presentation_image_h2/b3d02fc3da6af7a836ba563a5ecd718e/image-7.jpg)
Competitive inhibition [S]↑ inhibition↓
![Competitive inhibition ØVmax: unchanged* ØApparent Km (Kmapp, 表观Km): increased* 1/V [I] ↑ no I Competitive inhibition ØVmax: unchanged* ØApparent Km (Kmapp, 表观Km): increased* 1/V [I] ↑ no I](http://slidetodoc.com/presentation_image_h2/b3d02fc3da6af7a836ba563a5ecd718e/image-8.jpg)
Competitive inhibition ØVmax: unchanged* ØApparent Km (Kmapp, 表观Km): increased* 1/V [I] ↑ no I -1/Km 1/[S]

Competitive inhibition Ø I structure is similar to that of S Ø I only binds to free E and competes with S at active site Ø Vmax: unchanged Ø apparent Km (Kmapp, 表观Km): increased affinity of E for S is decreased Ø Inhibition is reversible as high[S] competes for I
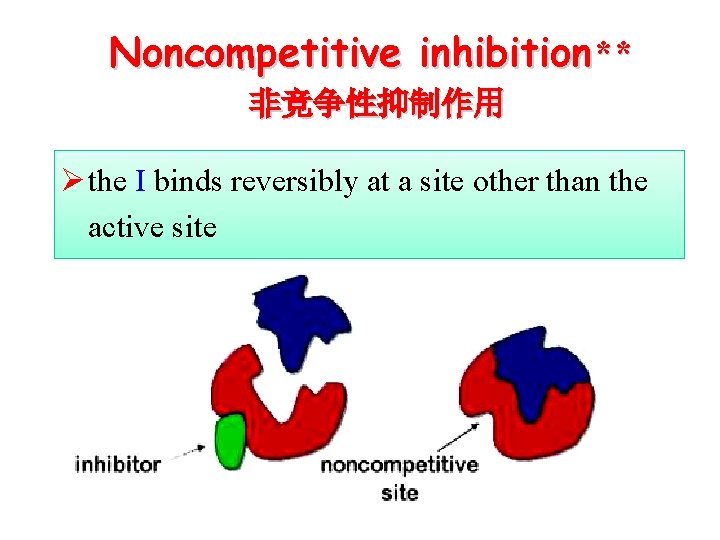
Noncompetitive inhibition** 非竞争性抑制作用 Ø the I binds reversibly at a site other than the active site
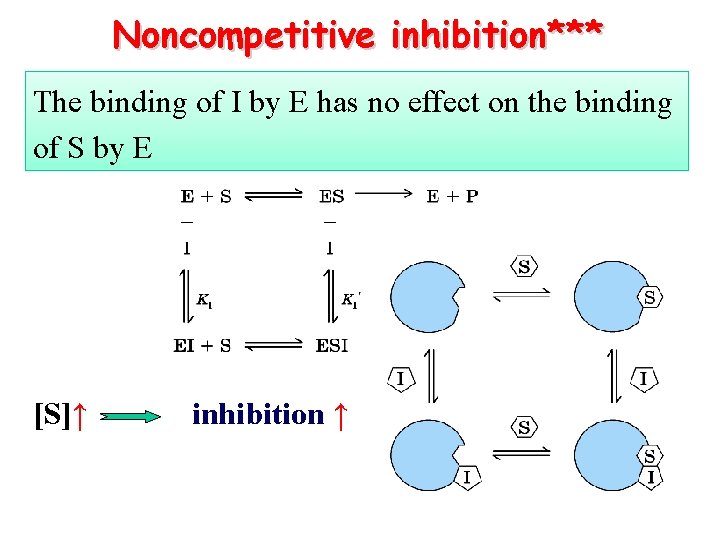
Noncompetitive inhibition*** The binding of I by E has no effect on the binding of S by E [S]↑ inhibition ↑
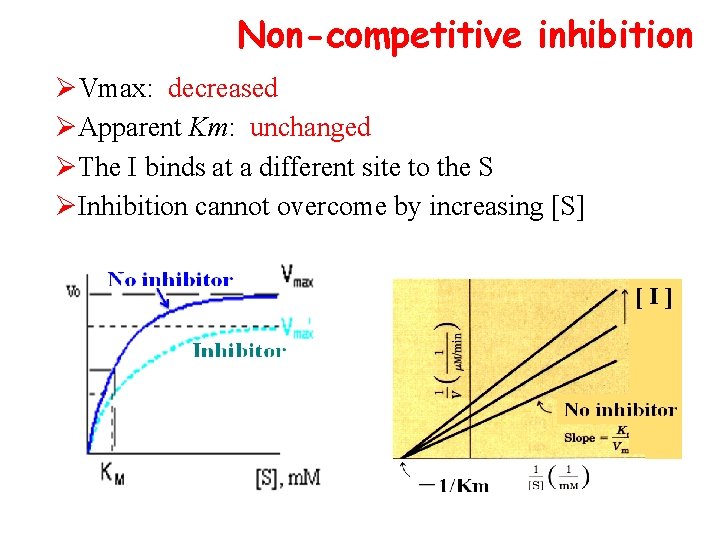
Non-competitive inhibition ØVmax: decreased ØApparent Km: unchanged ØThe I binds at a different site to the S ØInhibition cannot overcome by increasing [S]
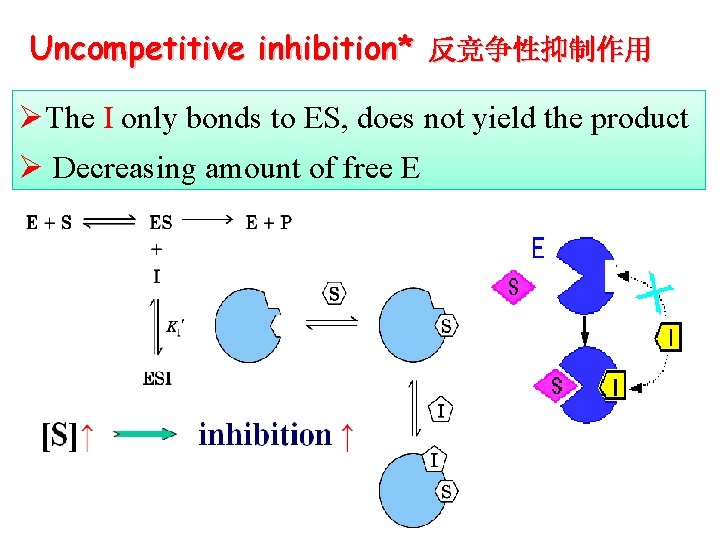
Uncompetitive inhibition* 反竞争性抑制作用 ØThe I only bonds to ES, does not yield the product Ø Decreasing amount of free E
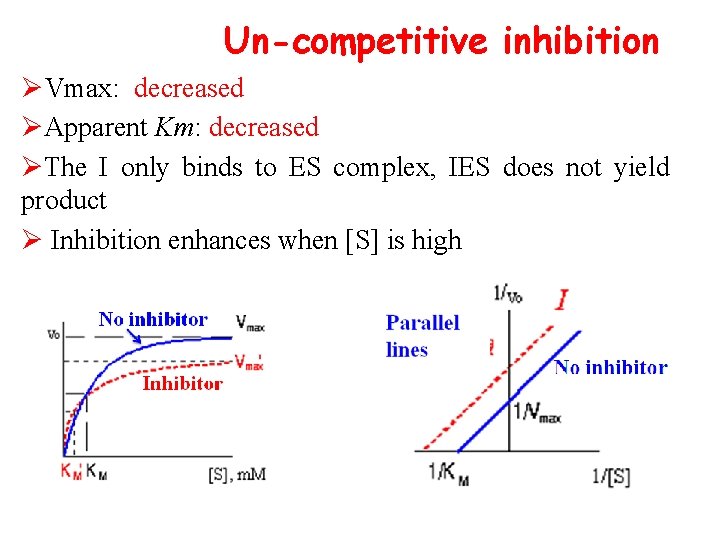
Un-competitive inhibition ØVmax: decreased ØApparent Km: decreased ØThe I only binds to ES complex, IES does not yield product Ø Inhibition enhances when [S] is high
![Competitive Noncompetitive Uncompetitive [S]↑ inhibition ↓ inhibition ↑ Competitive Noncompetitive Uncompetitive [S]↑ inhibition ↓ inhibition ↑](http://slidetodoc.com/presentation_image_h2/b3d02fc3da6af7a836ba563a5ecd718e/image-16.jpg)
Competitive Noncompetitive Uncompetitive [S]↑ inhibition ↓ inhibition ↑
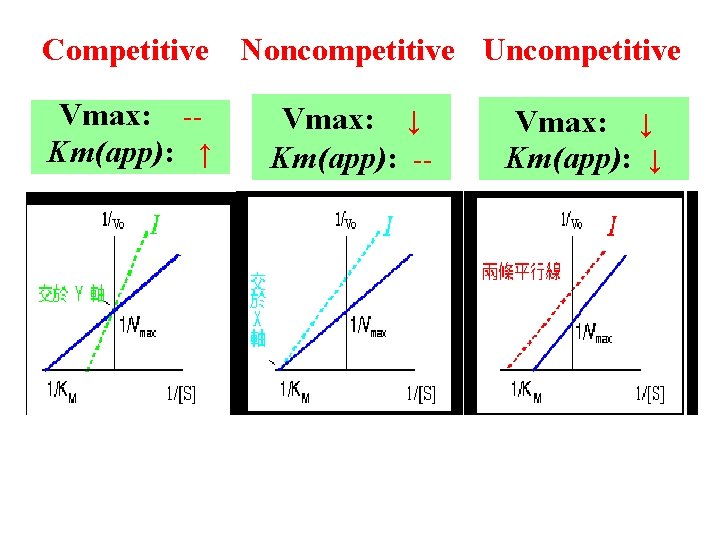
Competitive Noncompetitive Uncompetitive Vmax: -Km(app): ↑ Vmax: ↓ Km(app): -- Vmax: ↓ Km(app): ↓

Effect of activators on enzyme activity Activators increase enzyme activity Ø Some metal ions can increase E activity: Mg 2+, K+, Mn 2+, Ca 2+, Cl. Mg 2+ -- hexose kinase 己糖激酶 Ø Some compounds: bile salts 胆汁酸盐

Section IV Regualtion of Enzyme Activity n Regulation of enzyme amount (酶含量的调节) n Regulation of enzyme activity (酶活性的调节)
Fast control of enzyme activity 快速调节 Regulation of enzyme structure is a fast effect, including three mechanisms: ØAllosteric regulation*** 变构调节 binding of small molecules alters E structure Ø Covalent regulation*** 共价调节 covalent chemical modification changes E activity ØActivation of zymogen*** 酶原激活
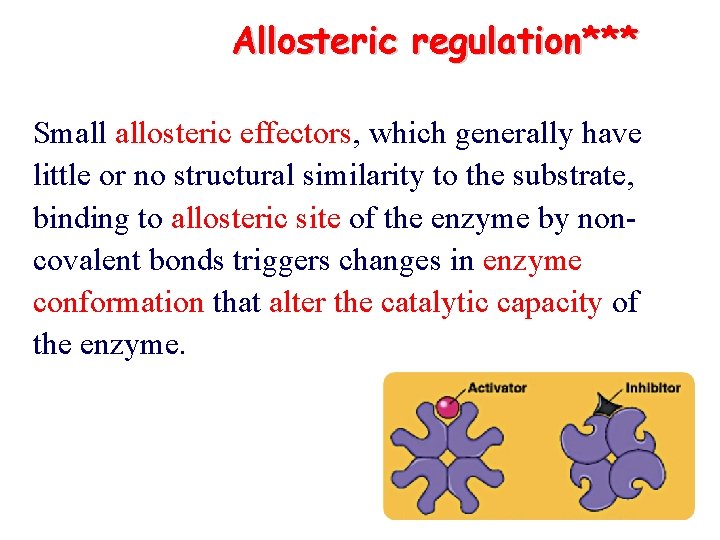
Allosteric regulation*** Small allosteric effectors, which generally have little or no structural similarity to the substrate, binding to allosteric site of the enzyme by noncovalent bonds triggers changes in enzyme conformation that alter the catalytic capacity of the enzyme.

Allosteric regulation ØAllosteric effectors: small molecules generally have little or no structural similarity to the substrate ØAllosteric site: allosteric effector binds ØCatalytic site: substrate binds ØThe allosteric site and catalytic site are distinct and separated spatially on enzyme
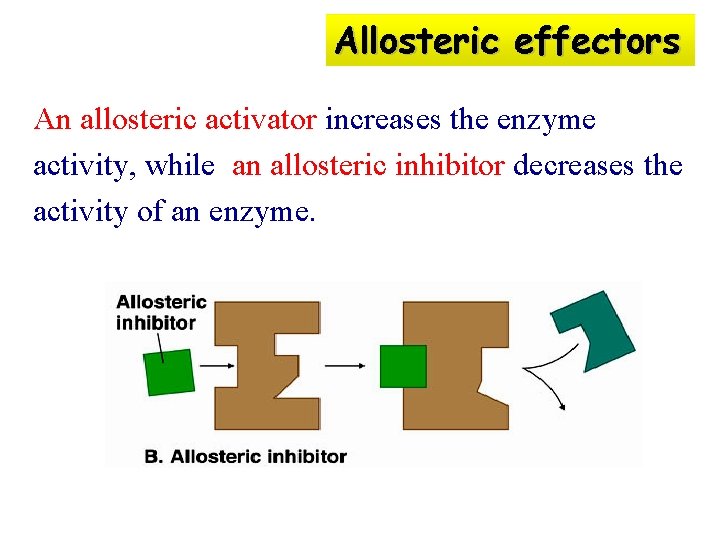
Allosteric effectors An allosteric activator increases the enzyme activity, while an allosteric inhibitor decreases the activity of an enzyme.
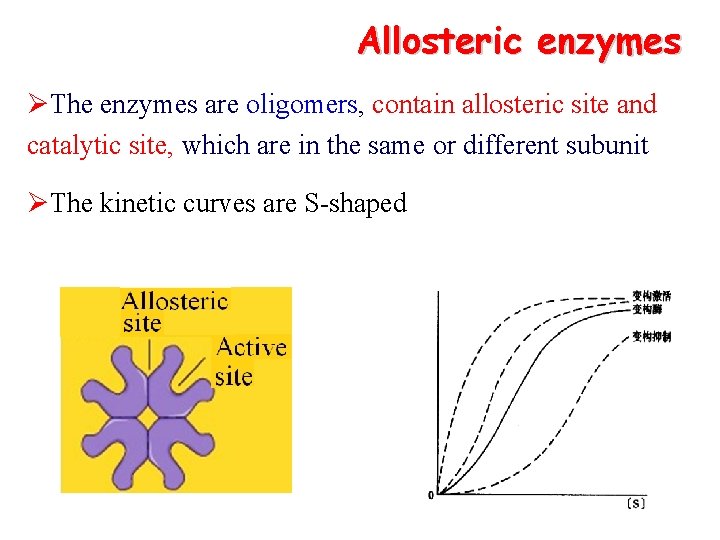
Allosteric enzymes ØThe enzymes are oligomers, contain allosteric site and catalytic site, which are in the same or different subunit ØThe kinetic curves are S-shaped

Covalent modification (共价修饰调节) Ø The structure and activity of many enzymes can be altered reversibly through covalent modification by another enzyme Ø Fast control of enzyme activity
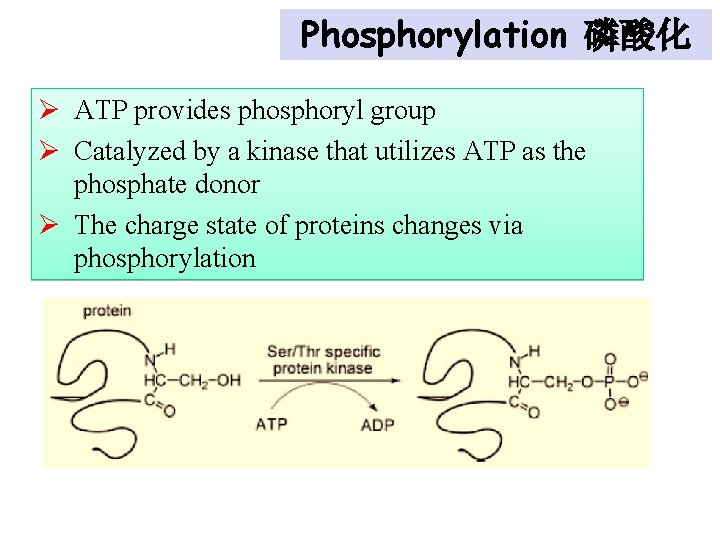
Phosphorylation 磷酸化 Ø ATP provides phosphoryl group Ø Catalyzed by a kinase that utilizes ATP as the phosphate donor Ø The charge state of proteins changes via phosphorylation
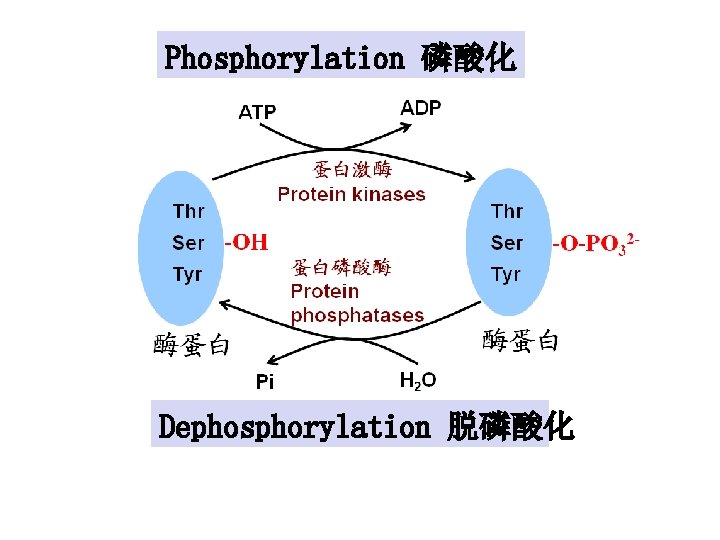
Phosphorylation 磷酸化 Dephosphorylation 脱磷酸化
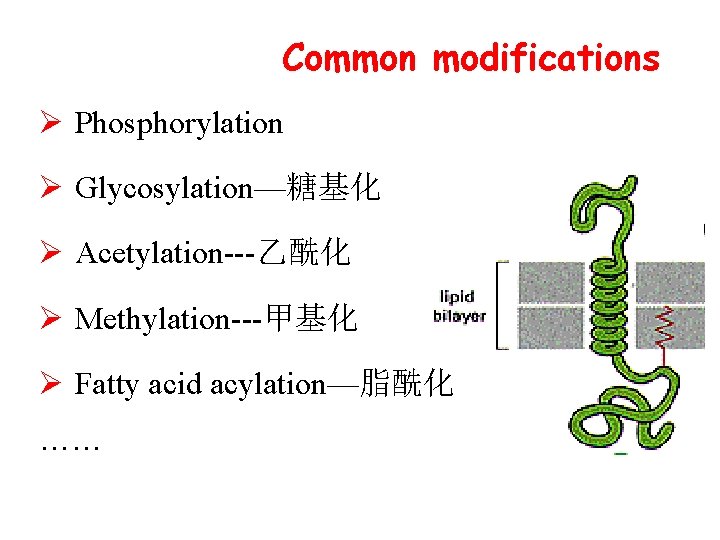
Common modifications Ø Phosphorylation Ø Glycosylation—糖基化 Ø Acetylation---乙酰化 Ø Methylation---甲基化 Ø Fatty acid acylation—脂酰化 ……

Zymogen and activation of zymogen 酶原与酶原激活

Zymogen: some enzymes are synthesized and secreted as large inactive precursors before exert their catalytic activity, called zymogens or proenzymes e. g. Trypsinogen 胰蛋白酶原 a digestive protease, is synthesized in pancreas and stomach, secreted into the digestive tract as zymogens

Zymogens The numerous of zymogens: (1) The digestive proteases 消化酶 (2) Enzymes involved in the blood clotting cascade (参与血液凝固级联效应的酶) (3) Plasmin (纤维溶解酶)
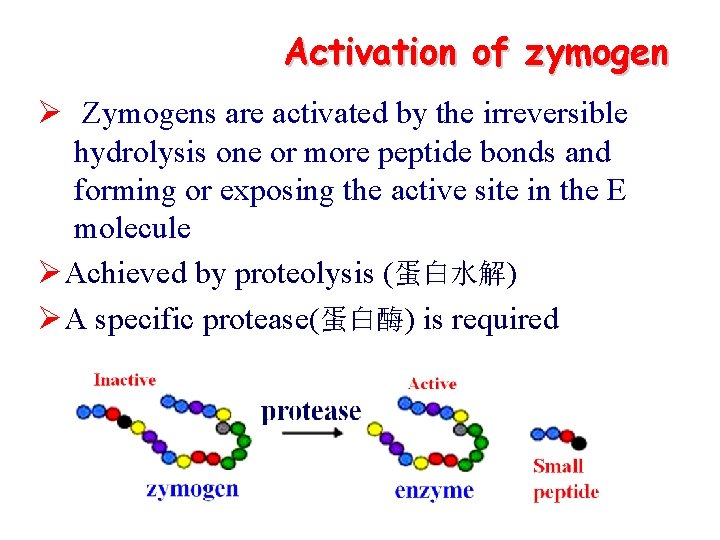
Activation of zymogen Ø Zymogens are activated by the irreversible hydrolysis one or more peptide bonds and forming or exposing the active site in the E molecule ØAchieved by proteolysis (蛋白水解) ØA specific protease(蛋白酶) is required
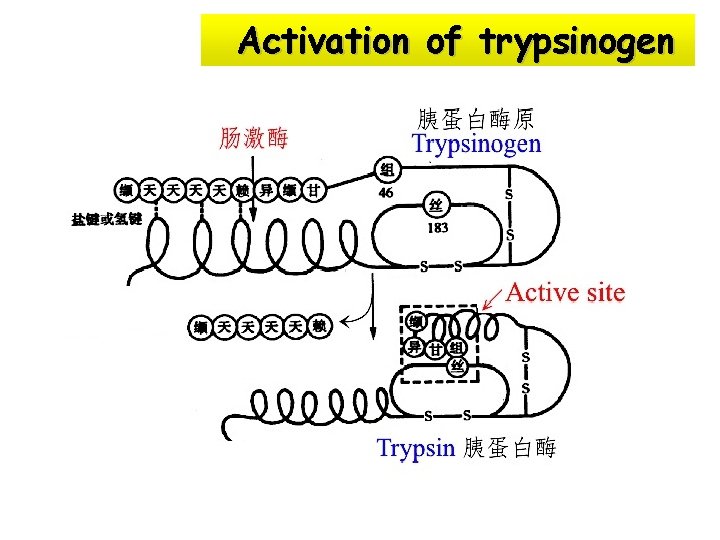
Activation of trypsinogen
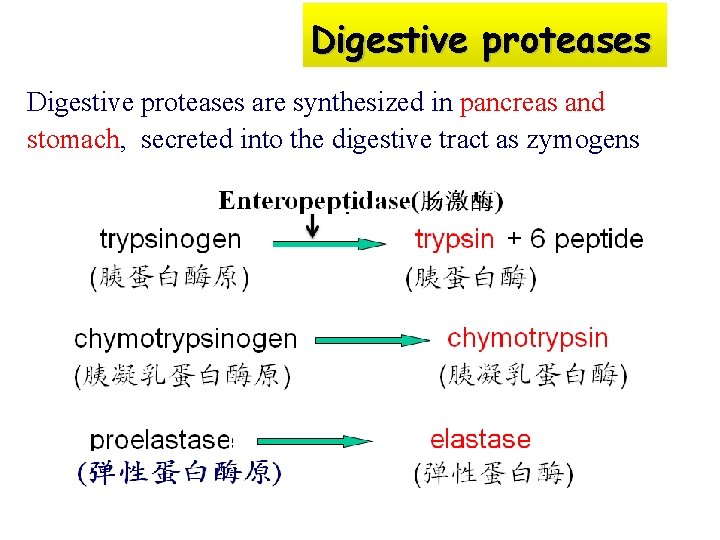
Digestive proteases are synthesized in pancreas and stomach, secreted into the digestive tract as zymogens

Physiological significances of zymogen activation (1) protection against their actions on its own tissue until need arises (2) Active enzymes have their catalytic functions in specific site and condition (3) As a storage form (for thrombinogen 凝血酶原, plasminogen纤溶酶原)
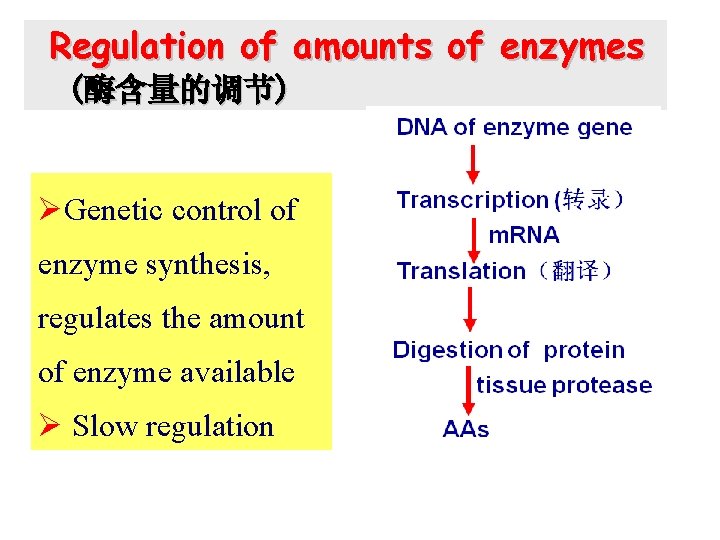
Regulation of amounts of enzymes (酶含量的调节) ØGenetic control of enzyme synthesis, regulates the amount of enzyme available Ø Slow regulation
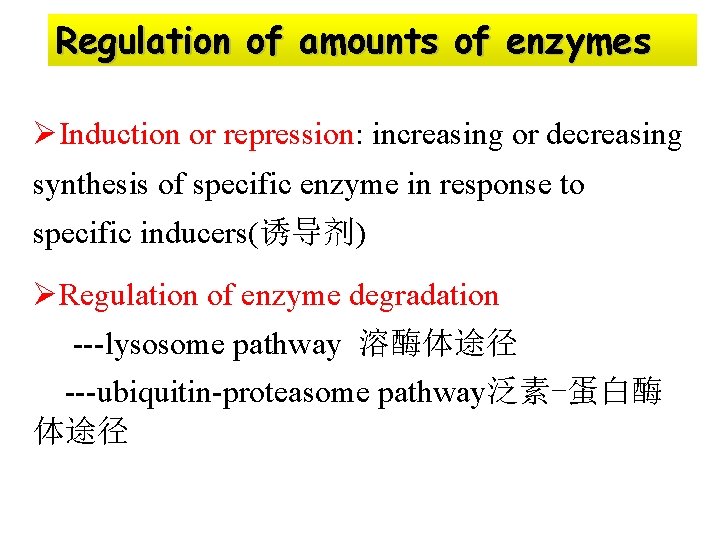
Regulation of amounts of enzymes ØInduction or repression: increasing or decreasing synthesis of specific enzyme in response to specific inducers(诱导剂) ØRegulation of enzyme degradation ---lysosome pathway 溶酶体途径 ---ubiquitin-proteasome pathway泛素-蛋白酶 体途径
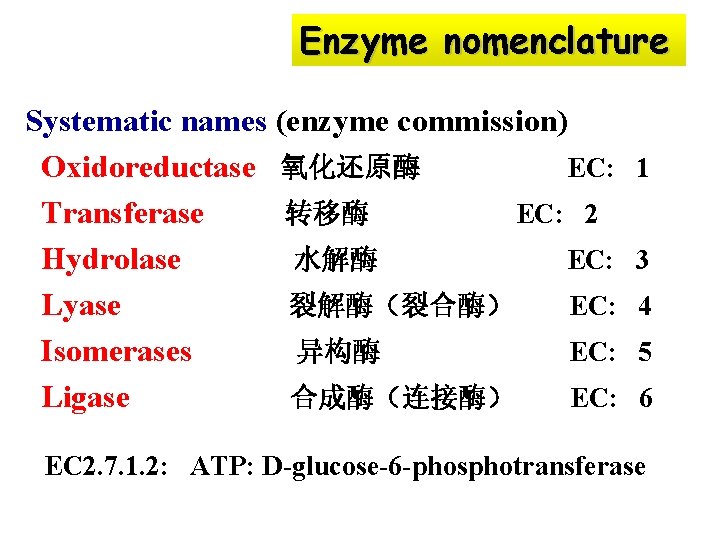
Enzyme nomenclature Systematic names (enzyme commission) Oxidoreductase 氧化还原酶 EC: Transferase 转移酶 EC: 2 Hydrolase 水解酶 EC: Lyase 裂解酶(裂合酶) EC: Isomerases 异构酶 EC: Ligase 合成酶(连接酶) EC: 1 3 4 5 6 EC 2. 7. 1. 2: ATP: D-glucose-6 -phosphotransferase
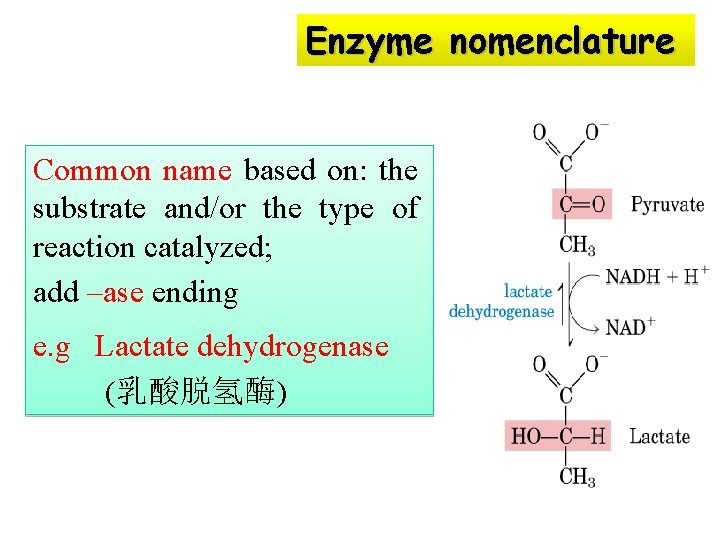
Enzyme nomenclature Common name based on: the substrate and/or the type of reaction catalyzed; add –ase ending e. g Lactate dehydrogenase (乳酸脱氢酶)
Summary ØEnzymes are proteins that alter other molecules ØEnzyme catalysis includes: --binding of the substrate in the active site -- catalysis: E lowers the activation energy by stabilizing the transition state; destabilizing the S; participating in the reaction -- release of altered S (product) Ø Reaction velocity depends on [S] and [E] --affinity of E and S, Km determined by M-M equation --Vmax determined by M-M equation

Summary ØEnzyme activity can be regulated by changed conformation, modified structure, and altered quantity -- p. H, temperature -- inhibitor, activator -- allosteric regulation by small molecules -- covalent modification via chemical groups -- activation of a zymogen by proteolysis

Summary Ø Some key questions: -- what is the active site? -- what is the activation energy? -- how to determine the affinity of a substrate for the active site in an enzyme? -- how the [S] affects the reaction velocity? what is Vmax? how is it determined? -- how to figure out a competitive inhibitor by the M-M curve? -- what is the active site, isozymes, zymogens. . .

Thank you
- Slides: 43