Effect of Aripiprazole Augmentation of Clozapine in Schizophrenia









- Slides: 28

Effect of Aripiprazole Augmentation of Clozapine in Schizophrenia: A Doubleblind, Placebo-controlled Study Journal Club Psychiatry rotation

Background ¡ 15 -20% of patients have poor outcome, treatment resistant ¡ 30 -50% of treatment resistant patients only partially responsive to clozapine ¡ Lack of evidence on efficacy & tolerability of combination treatment with clozapine ¡ Case reports, open-label studies & case series on clozapine + aripiprazole: promising therapeutic strategy in residual & treatment-resistant patients

Clozapine ¡ Weak antagonist at: l ¡ ¡ D 1, D 2, D 3, and D 5 Antagonist at D 4: High affinity Antagonist at 5 -HT 2 A, alphaadrenergic, H 1& cholinergic receptors

Aripiprazole ¡ 2 nd generation APs: High 5 HT 2: D 2 affinity ratio, lower affinity for D 2 ¡ Aripiprazole: Low 5 HT 2: D 2 affinity ratio, higher affinity for D 2 ¡ Partial agonist at pre & post synaptic D 2 receptors hypothesized to exert: l l Functional antagonist in a hyperdopaminergic environment Functional agonist in a hypodopaminergic environment

Aripiprazole ¡ Partial agonist: 5 -HT 1 A ¡ Antagonist at 5 -HT 2 A receptors in mesocortical tract l postulated to ↑ dopamine release and ↓ negative symptoms ¡ Comparable efficacy to other antipsychotics for +ve symptoms. ¡ May be beneficial for cognitive, negative & mood symptoms

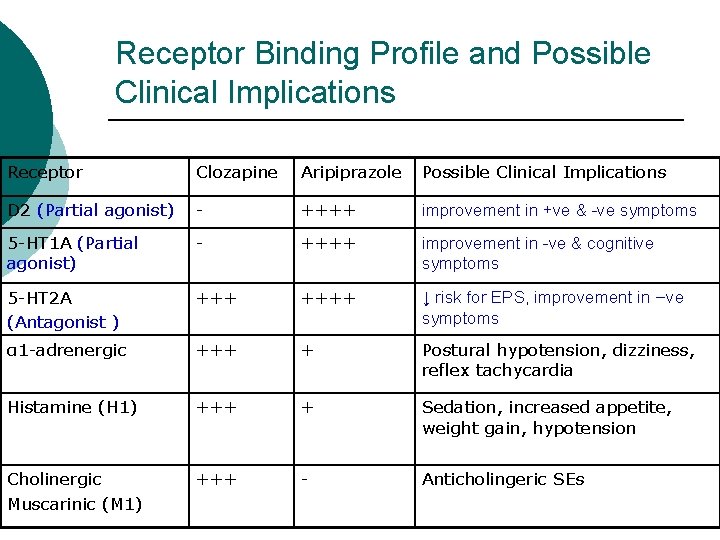
Receptor Binding Profile and Possible Clinical Implications Receptor Clozapine Aripiprazole Possible Clinical Implications D 2 (Partial agonist) - ++++ improvement in +ve & -ve symptoms 5 -HT 1 A (Partial agonist) - ++++ improvement in -ve & cognitive symptoms 5 -HT 2 A (Antagonist ) ++++ ↓ risk for EPS, improvement in –ve symptoms α 1 -adrenergic +++ + Postural hypotension, dizziness, reflex tachycardia Histamine (H 1) +++ + Sedation, increased appetite, weight gain, hypotension Cholinergic Muscarinic (M 1) +++ - Anticholingeric SEs

Pharmacokinetics ¡ F = 87% ¡ Mean T 1/2 = 75 hrs ¡ Mean Tmax = 3. 0 hrs ¡ Time to steady state ~ 14 days ¡ Dose proportional Cmax & AUC b/w 5 mg and 30 mg daily ¡ No dose adjustment for renal or hepatic insufficiency

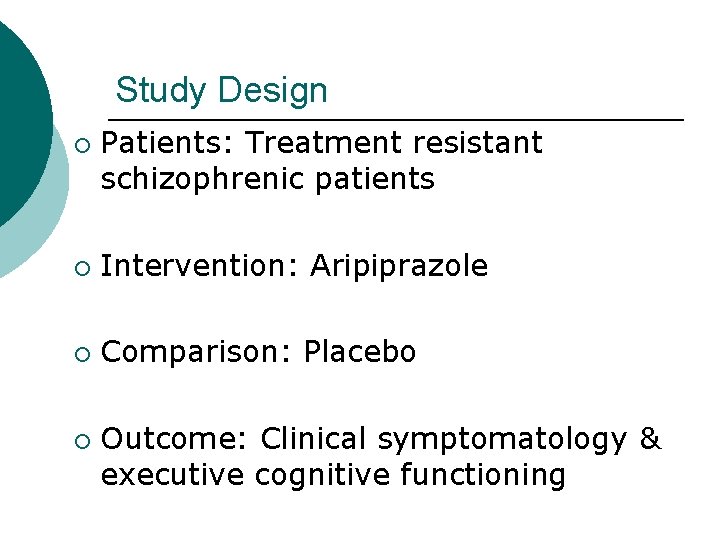
Study Design ¡ Patients: Treatment resistant schizophrenic patients ¡ Intervention: Aripiprazole ¡ Comparison: Placebo ¡ Outcome: Clinical symptomatology & executive cognitive functioning

Study Design ¡ Randomized, double-blind, placebo-controlled ¡ Until Week 12: 10 mg/day ¡ After Week 12: 15 mg/ day ¡ 5 mg/day of lorazepam allowed for insomnia or agitation

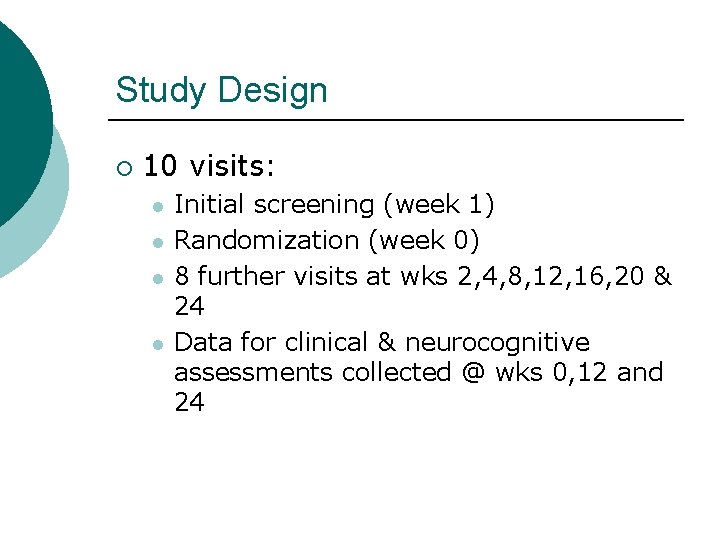
Study Design ¡ 10 visits: l l Initial screening (week 1) Randomization (week 0) 8 further visits at wks 2, 4, 8, 12, 16, 20 & 24 Data for clinical & neurocognitive assessments collected @ wks 0, 12 and 24

Inclusion Criteria ¡ ¡ ¡ Met DSM-IV criteria for schizophrenia Positive & negative symptoms despite an adequate trial of clozapine Brief Psychiatric Rating Scale: >25 partial-responders or non-responders

Exclusion Criteria ¡ ¡ ¡ ¡ Any major psychiatric disorder Significant concurrent medical illnesses Organic brain disorder Hx of substance & alcohol abuse Mental retardation Pregnant or lactating women No Anti-Depressant or Anti-Convulsant for 2 months before study

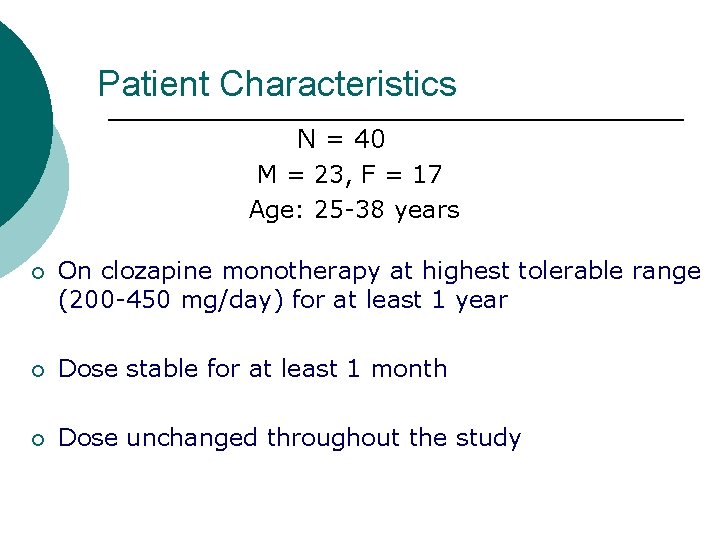
Patient Characteristics N = 40 M = 23, F = 17 Age: 25 -38 years ¡ On clozapine monotherapy at highest tolerable range (200 -450 mg/day) for at least 1 year ¡ Dose stable for at least 1 month ¡ Dose unchanged throughout the study

Scales Used to Test Efficacy (Psychopathological) ¡ ¡ BPRS: Brief Psychiatric Rating Scale SANS: Scale for the Assessment of Negative Symptoms SAPS: Scale for the Assessment of Positive Symptoms CDSS: Calgary Depression Scale for Schizophrenia

Scales Used to Test Efficacy (Neurocognitive) ¡ WCST: Wisconsin Card Sorting System ¡ Verbal Fluency Test ¡ Stroop Colour-word Test

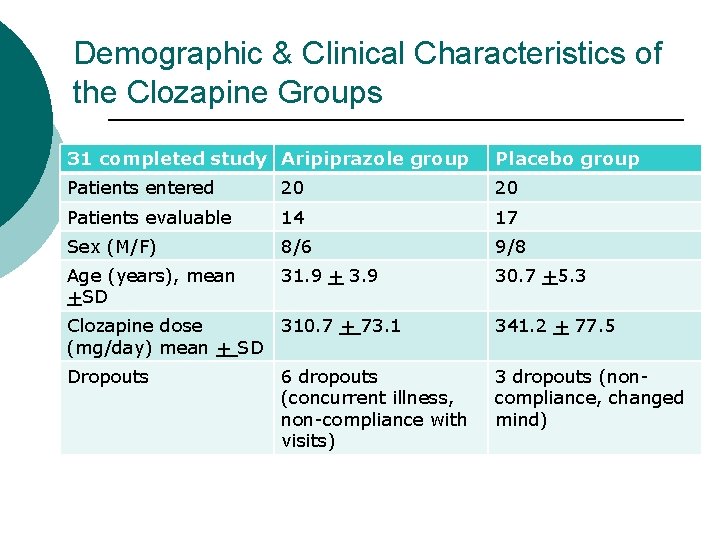
Demographic & Clinical Characteristics of the Clozapine Groups 31 completed study Aripiprazole group Placebo group Patients entered 20 20 Patients evaluable 14 17 Sex (M/F) 8/6 9/8 Age (years), mean +SD 31. 9 + 3. 9 30. 7 +5. 3 Clozapine dose 310. 7 + 73. 1 (mg/day) mean + SD 341. 2 + 77. 5 Dropouts 3 dropouts (noncompliance, changed mind) 6 dropouts (concurrent illness, non-compliance with visits)

Lorazepam Use for Insomnia or Agitation ¡ Aripiprazole group: l l ¡ Placebo group: l l ¡ Patient 1 = 2. 5 mg/day Patient 2 = 5 mg/day Patient 1, 2 = 2. 5 mg/day Patient 3 = 5 mg/day Small N, no statistical analyses performed

Results

Results

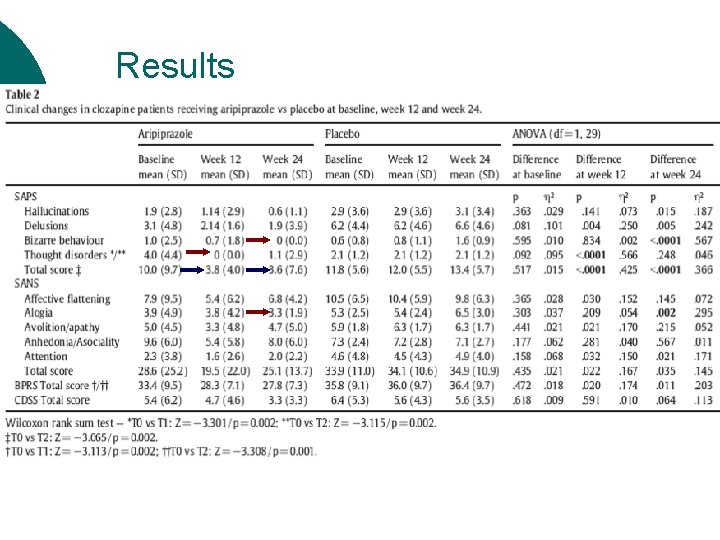
Results ¡ Positive symptoms: Aripiprazole > Placebo l l ¡ SAPS total scores Domains delusions & bizarre behaviour Negative symptoms: Aripiprazole > Placebo l l l Single domain of alogia Lower reduction than expected Mild negative symptoms ¡ ↑ in overall psychopathological state: Changes in BPRS ¡ Affective symptomatology: No changes in CDSS

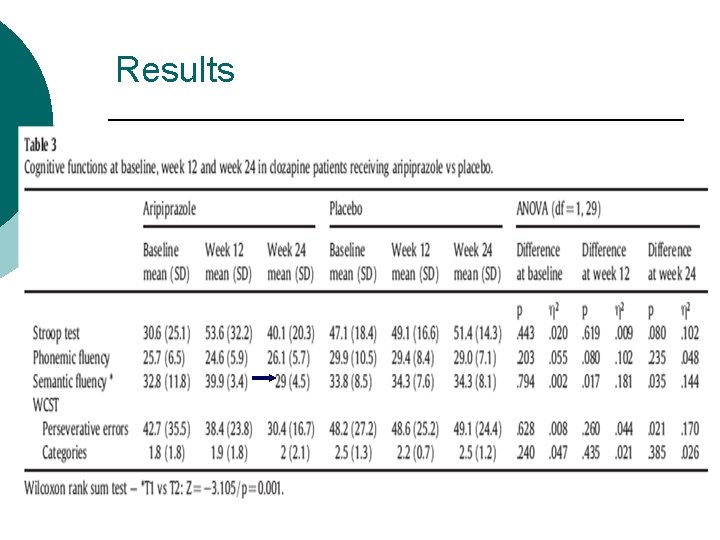
Results ¡ Positive & general psychopathological symptomatology: Beneficial effect ¡ Executive cognitive functions: No significant effects ¡ ¡ Safety: generally well-tolerated Most common SEs: restlessness (N=5, 35. 7%), insomnia (N=3, 21. 4%), nausea (N =1, 7. 1%)

Results from other studies ¡ Double-blind RCT (Chang et al. ): No advantage for total symptom severity ¡ Secondary analyses: Significant ↑ in negative symptoms and overall clinical state (BPRS scores) ¡ Limited evidence on cognition ¡ Open label RCT, N= 169 l l ¡ ↑ in general cognitive functioning Significant ↑ in verbal learning Case report: ↑ in verbal memory, reaction time, quality/attention

Investigators’ Conclusion ¡ ¡ ¡ Combination well-tolerated May be of benefit for patients partially responsive to clozapine monotherapy Further double-blind, placebo controlled trials in a larger number of patients required

Critical Appraisal Skills Programme (CASP) RCT Checklist ¡ Did the study ask a clearly focused question? Yes ¡ Was this a randomized controlled trial (RCT) and was it appropriately so? Yes ¡ Were participants appropriately allocated to intervention and control groups? Yes ¡ Were participants, staff and study personnel ‘blind’ to participants’ study group? Yes ¡ Were all of the participants who entered the trial accounted for at its conclusion? Yes

Critical Appraisal Skills Programme (CASP) RCT Checklist ¡ Were the participants in all groups followed up and data collected in the same way? Yes ¡ Did the study have enough participants to minimize the play of chance? No ¡ How are the results presented and what is the main result? Augmentation beneficial for on positive & general psychopathological symptomatology l No significant effects regarding executive cognitive functions l ¡ How precise are these results? ¡ Were all important outcomes considered so the results can be applied? Concurrent medical conditions, medications

Limitations ¡ Small sample size ¡ Relatively low dose of aripiprazole l May have prevented enhanced therapeutic effects l No discussion regarding biphasic titration ¡ Practice effects ¡ No information on clozapine levels ¡ Patient status: smoker vs. non-smoker

Limitations ¡ SEs data: l No data regarding metabolic SEs l Clinical interview l Non-specific questioning l No formal psychometric measure of EPS ¡ Inter-rater reliability not established by formal training

Implications to Practice ¡ ¡ ¡ Polypharmacy not the best option in terms of antipsychotics Trial in patients with partial response to clozapine More RCTs required