EEA Grants Norway Grants Introduction to kinetics and

![Rate laws • Differential rate law (Guldberg-Waag low): v = k[A]a[B]b[C]c k ………… rate Rate laws • Differential rate law (Guldberg-Waag low): v = k[A]a[B]b[C]c k ………… rate](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-8.jpg)
![Zero-order reactions A P [k] = mol dm-3 s-1 Zero-order reactions A P [k] = mol dm-3 s-1](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-10.jpg)
![First order reactions A→P [k] = s-1 First order reactions A→P [k] = s-1](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-13.jpg)


![Second order reactions 2 A → P [k] = dm 3 mol-1 s-1 Second order reactions 2 A → P [k] = dm 3 mol-1 s-1](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-18.jpg)
![Second order reactions A+B→P [k] = dm 3 mol-1 s-1 Second order reactions A+B→P [k] = dm 3 mol-1 s-1](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-21.jpg)
![Summary Charact kinetic plot Scope of kinetic plot Units of rate constant Zero [A] Summary Charact kinetic plot Scope of kinetic plot Units of rate constant Zero [A]](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-22.jpg)




- Slides: 42

EEA Grants Norway Grants Introduction to kinetics and catalysis Ing. Marcela Králová Ph. D. , CEITEC 20. 4. 2015

Content • Kinetics • Reaction lows • Reactions orders and its determination • Theory of chemical reactions • Homogeneous catalysis • Heterogeneous catalysis • Photocatalysis

Kinetics • Deal with the rates of chemical processes • Chemical processes – sequence of one or more single step • Elementary process – transition between two atomic/molecular state separated by a potential barrier • Activation energy • Low barrier = fast reaction • High barrier = slow reaction • Elementary reactions • Single reactive collision (bimolecular step) • Dissociations/isomerisation (unimol. step) • Termolecular step • Goals of the study: • Reaction mechanisms • Absolute reaction rate

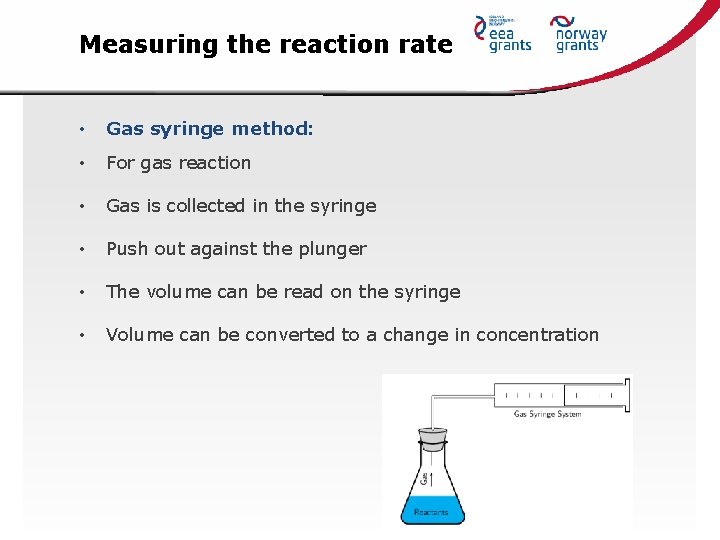
Measuring the reaction rate • Gas syringe method: • For gas reaction • Gas is collected in the syringe • Push out against the plunger • The volume can be read on the syringe • Volume can be converted to a change in concentration

Measuring the reaction rate • Changes in mass: • For gas reaction • Calculation of mass loss • Gas escapes from the reaction flask • • Mass of precipitation: For reaction where the precipitation is formed • Using stopwatch

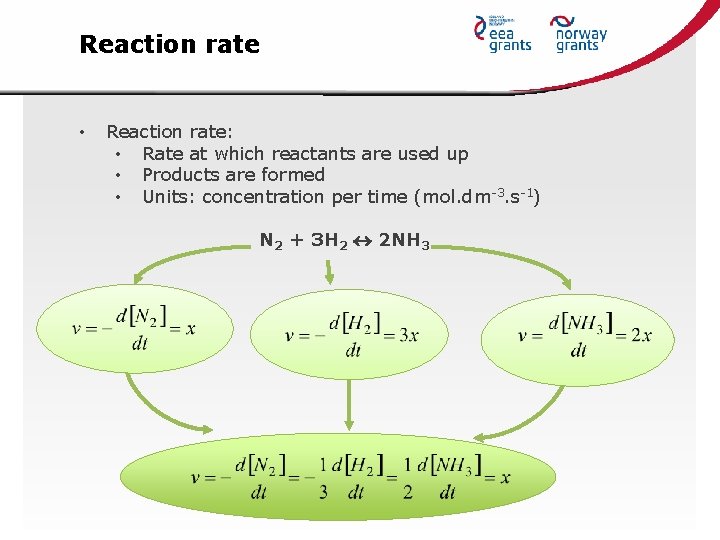
Reaction rate • Reaction rate: • Rate at which reactants are used up • Products are formed • Units: concentration per time (mol. dm-3. s-1) N 2 + 3 H 2 2 NH 3

Rate laws • • Differential rate low: • Changes of reaction rate with the concentration • Reaction rate is proportional to the rate of conc. changes • Rate is proportional to derivative of concentration Integrated rate low: • Relates the concentration to time
![Rate laws Differential rate law GuldbergWaag low v kAaBbCc k rate Rate laws • Differential rate law (Guldberg-Waag low): v = k[A]a[B]b[C]c k ………… rate](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-8.jpg)
Rate laws • Differential rate law (Guldberg-Waag low): v = k[A]a[B]b[C]c k ………… rate constant powers…………. . partial order of the reaction with respect to the reactant overall order…. . sum of the powers N 2 + 3 H 2 2 NH 3

Zero-order reactions • Rate is independent on the concentration of the reactant • Examples: • some photochemical • enzymatic catalyzed reactions • reverse Haber process: 2 NH 3(g) 3 H 2(g) + N 2(g)
![Zeroorder reactions A P k mol dm3 s1 Zero-order reactions A P [k] = mol dm-3 s-1](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-10.jpg)
Zero-order reactions A P [k] = mol dm-3 s-1

Zero-order reactions • Half-life 1/2: • Required time for half of the reactants to be depleted

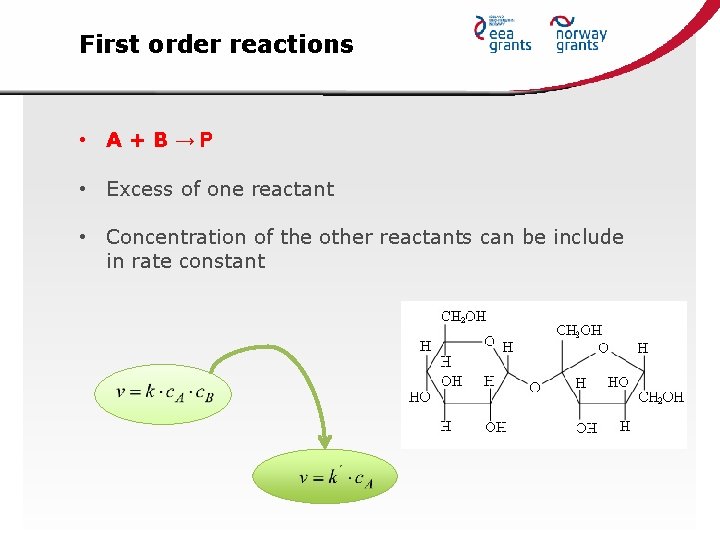
First order reactions • Rate is dependent on the concentration of one reactant • Other reactant can be present, but each will be zero-order • Examples: • A P • A+B P; where one component is in excess
![First order reactions AP k s1 First order reactions A→P [k] = s-1](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-13.jpg)
First order reactions A→P [k] = s-1

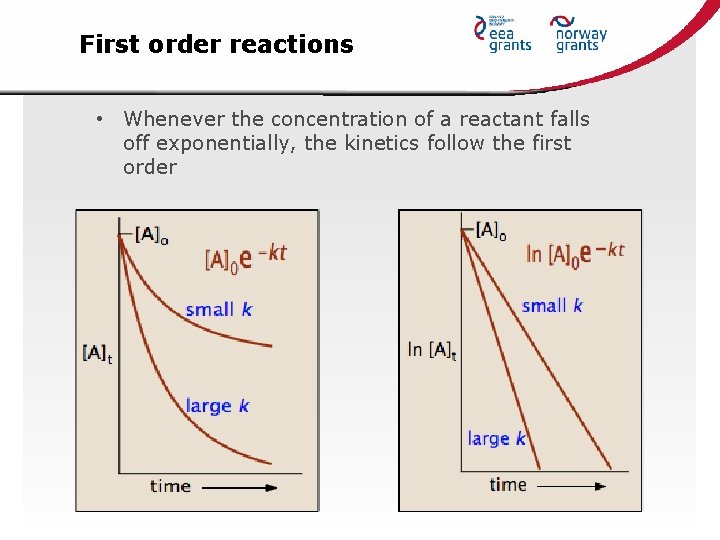
First order reactions • Whenever the concentration of a reactant falls off exponentially, the kinetics follow the first order

First order reactions • Half-life 1/2:

First order reactions • A+B→P • Excess of one reactant • Concentration of the other reactants can be include in rate constant

Second order reactions • Rate is dependent on the concentration of • one second-order reactant (2 A P) • two first order reactants (A+B P)
![Second order reactions 2 A P k dm 3 mol1 s1 Second order reactions 2 A → P [k] = dm 3 mol-1 s-1](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-18.jpg)
Second order reactions 2 A → P [k] = dm 3 mol-1 s-1

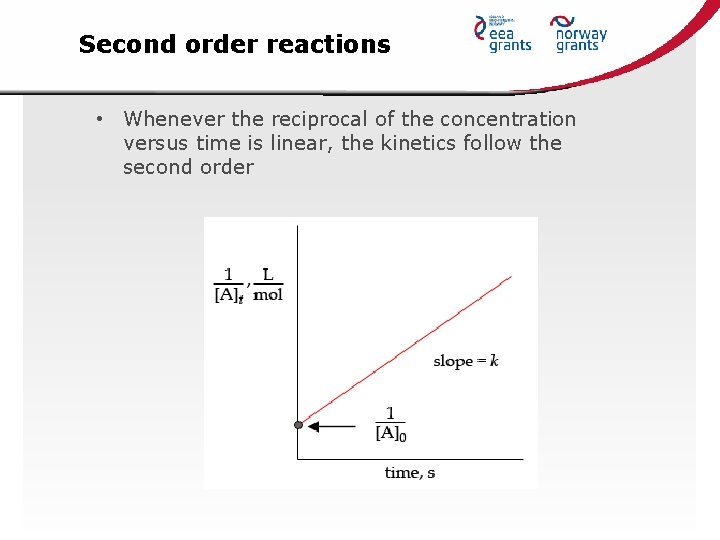
Second order reactions • Whenever the reciprocal of the concentration versus time is linear, the kinetics follow the second order

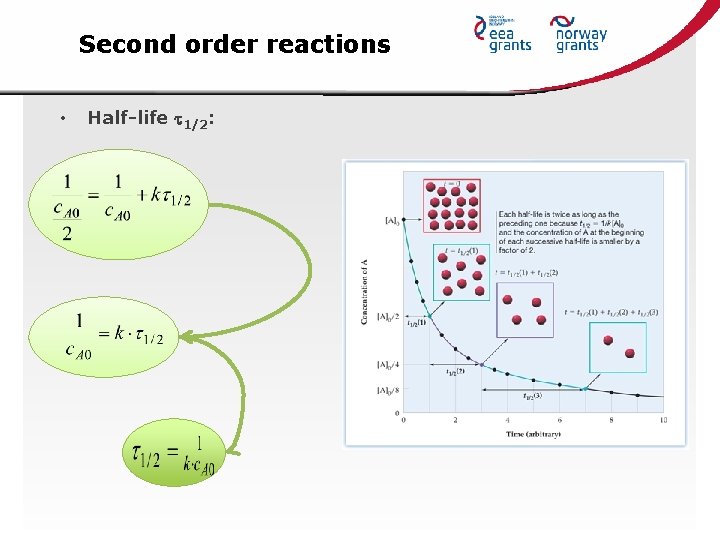
Second order reactions • Half-life 1/2:
![Second order reactions ABP k dm 3 mol1 s1 Second order reactions A+B→P [k] = dm 3 mol-1 s-1](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-21.jpg)
Second order reactions A+B→P [k] = dm 3 mol-1 s-1
![Summary Charact kinetic plot Scope of kinetic plot Units of rate constant Zero A Summary Charact kinetic plot Scope of kinetic plot Units of rate constant Zero [A]](https://slidetodoc.com/presentation_image_h/deccfc841a4e7874fe7c54e865f22893/image-22.jpg)
Summary Charact kinetic plot Scope of kinetic plot Units of rate constant Zero [A] vs t -k mol dm-3 s First ln[A] vs t -k s-1 k dm 3 mol-1 s-1 Reaction order Second Differntial rate law Integrated rate low 1/[A] vs t -1

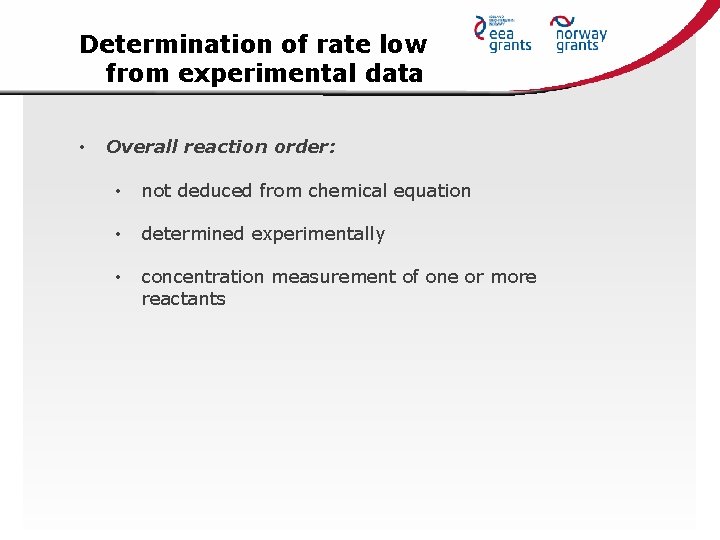
Determination of rate low from experimental data • Overall reaction order: • not deduced from chemical equation • determined experimentally • concentration measurement of one or more reactants

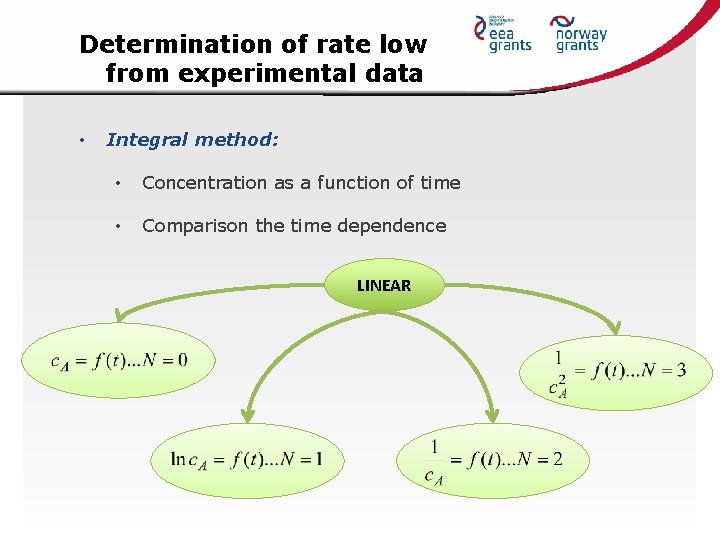
Determination of rate low from experimental data • Integral method: • Concentration as a function of time • Comparison the time dependence LINEAR

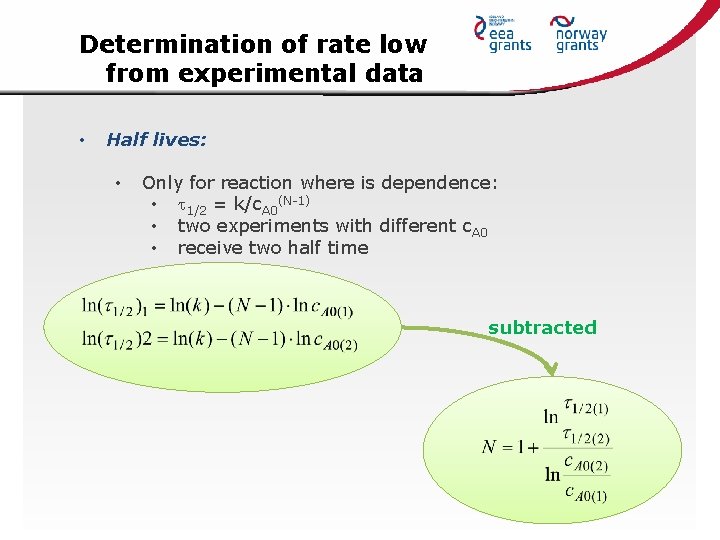
Determination of rate low from experimental data • Half lives: • Only for reaction where is dependence: • 1/2 = k/c. A 0(N-1) • two experiments with different c. A 0 • receive two half time subtracted

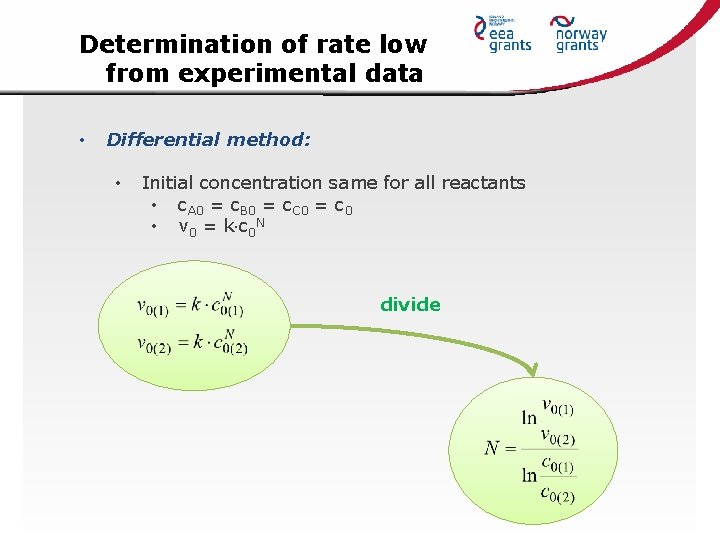
Determination of rate low from experimental data • Differential method: • Initial concentration same for all reactants • c. A 0 = c. B 0 = c. C 0 = c 0 • v 0 = k c 0 N divide

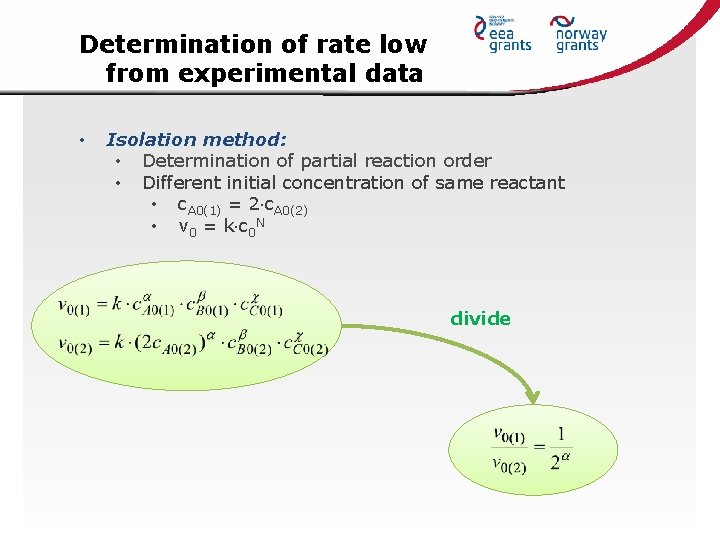
Determination of rate low from experimental data • Isolation method: • Determination of partial reaction order • Different initial concentration of same reactant • c. A 0(1) = 2 c. A 0(2) • v 0 = k c 0 N divide

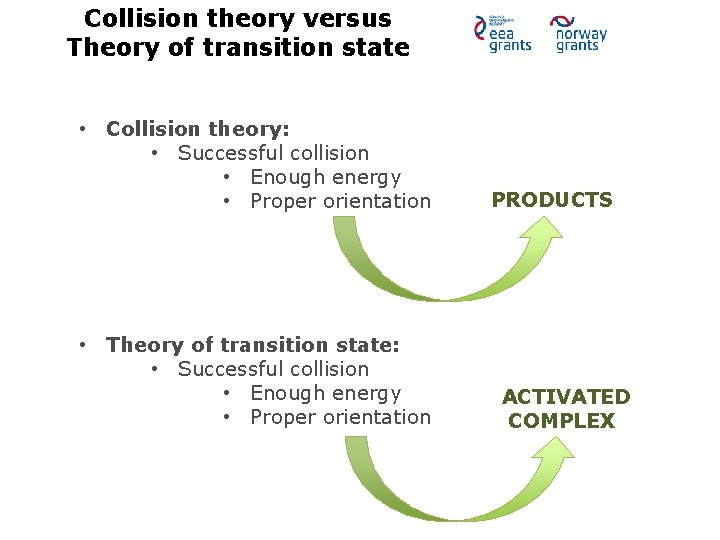
Collision theory • Explain: • How chemical reaction occur • Why reaction rate is different for different reaction • Criteria: • Sufficient kinetic energy (activation energy) • Proper orientation • Sufficient collision

Collision theory A + B → C • A and B are gasses • Frequency of collision is proportional to the concentration of A and B • Doubling of c. A, the frequency of A-B collision double • The rate at witch molecules collide affect the overall reaction rate

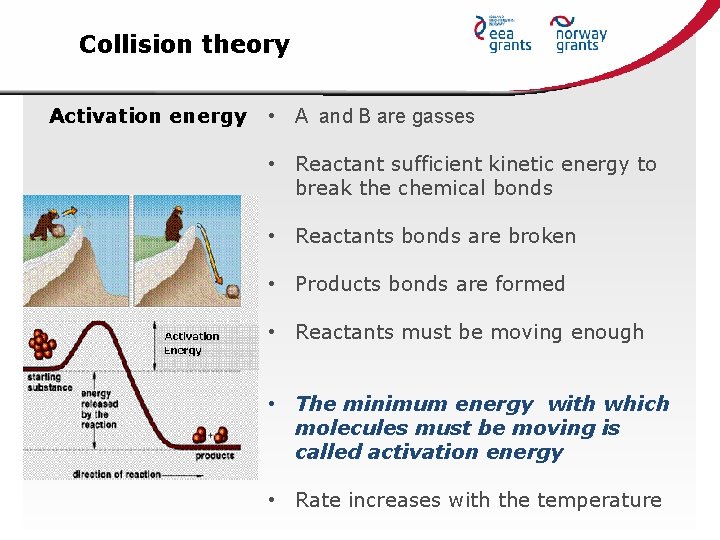
Collision theory Activation energy • A and B are gasses • Reactant sufficient kinetic energy to break the chemical bonds • Reactants bonds are broken • Products bonds are formed • Reactants must be moving enough • The minimum energy with which molecules must be moving is called activation energy • Rate increases with the temperature

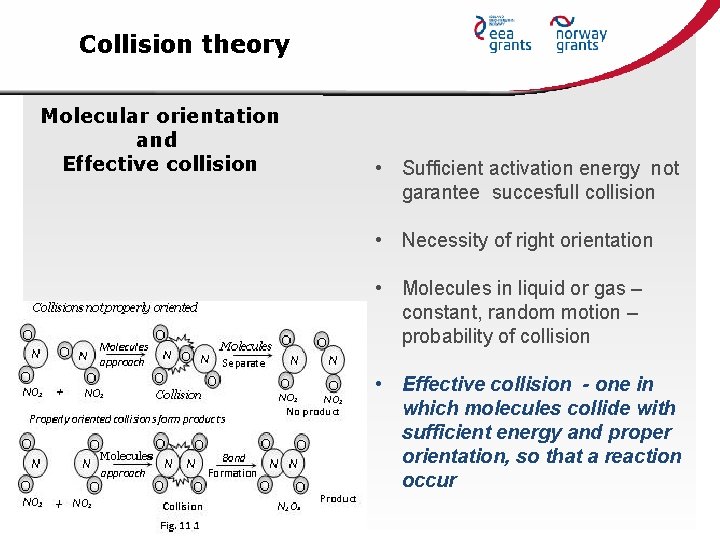
Collision theory Molecular orientation and Effective collision • Sufficient activation energy not garantee succesfull collision • Necessity of right orientation • Molecules in liquid or gas – constant, random motion – probability of collision • Effective collision - one in which molecules collide with sufficient energy and proper orientation, so that a reaction occur

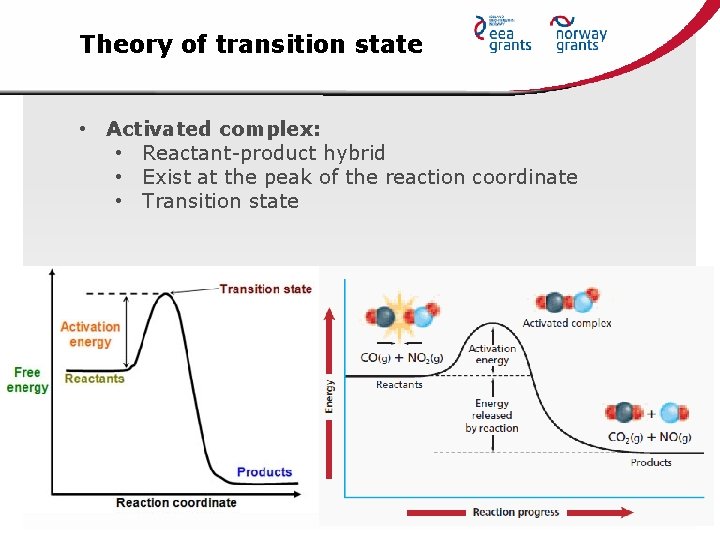
Theory of transition state • Postulate the existence of hypothetical transition state • It occurs between reactants and products state • Formed species is called activated complex • Based upon collision theory

Theory of transition state • Activated complex: • Reactant-product hybrid • Exist at the peak of the reaction coordinate • Transition state

Theory of transition state • Factors determines if the reaction occur or not: • Concentration of the activated complex • The rate at which the activated complex breaks apart • The mechanism by which the activated complex breaks apart • Back to reactants • Towards products

Collision theory versus Theory of transition state • Collision theory: • Successful collision • Enough energy • Proper orientation • Theory of transition state: • Successful collision • Enough energy • Proper orientation PRODUCTS ACTIVATED COMPLEX

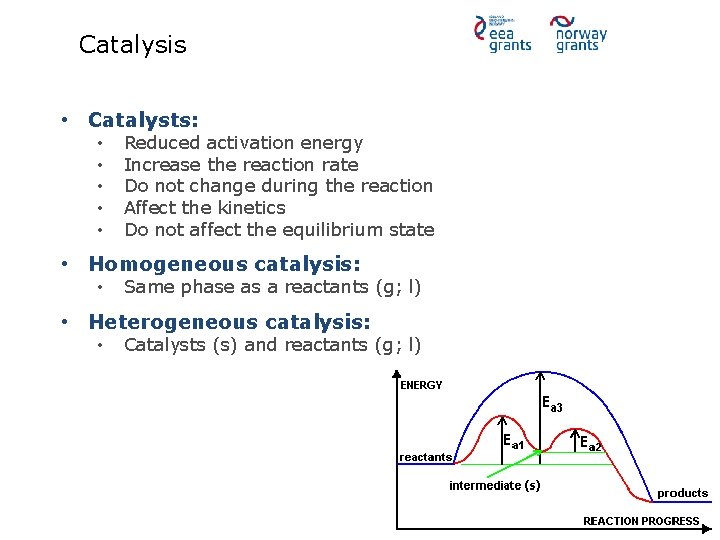
Catalysis • Catalysts: • • • Reduced activation energy Increase the reaction rate Do not change during the reaction Affect the kinetics Do not affect the equilibrium state • Homogeneous catalysis: • Same phase as a reactants (g; l) • Heterogeneous catalysis: • Catalysts (s) and reactants (g; l)

Homogeneous catalysis • Examples: • • • Acid catalysis Organometallic catalysis Enzymatic catalysis • Advantage: • • Mix into the reaction mixture High degree of interaction: catalyst-reactant • Disadvantage: • Irrecoverable after the reaction

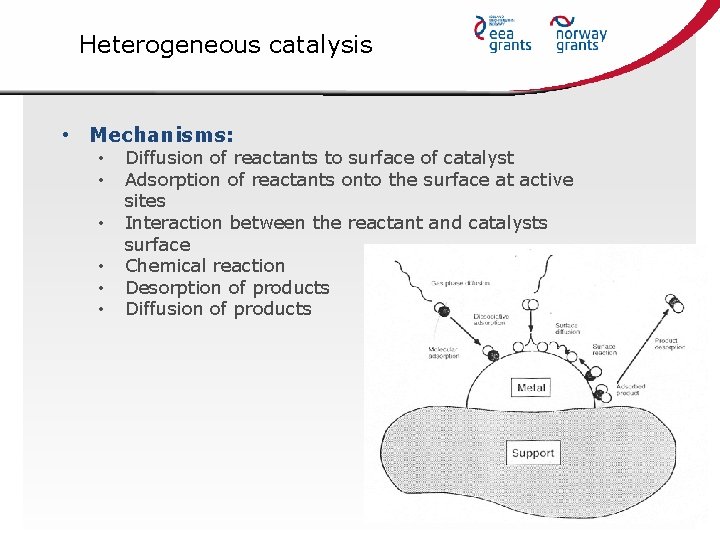
Heterogeneous catalysis • Mechanisms: • • • Diffusion of reactants to surface of catalyst Adsorption of reactants onto the surface at active sites Interaction between the reactant and catalysts surface Chemical reaction Desorption of products Diffusion of products

Heterogeneous catalysis • Advantage: • Separation form the reaction mixture • Disadvantage: • Saturation of catalyst surface

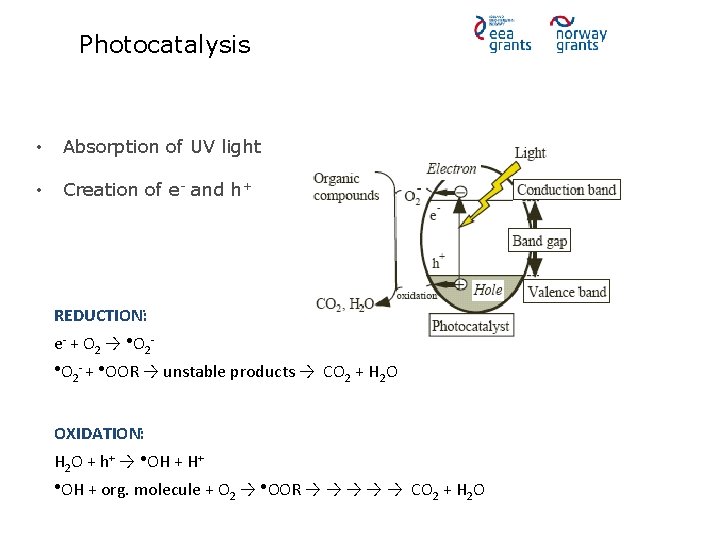
Photocatalysis • Absorption of UV light • Creation of e- and h+ REDUCTION: e- + O 2 → ●O 2●O - + ●OOR 2 → unstable products → CO 2 + H 2 O OXIDATION: H 2 O + h+ → ●OH + H+ ●OH + org. molecule + O 2 → ●OOR → → → CO 2 + H 2 O

Photocatalysis • Ti. O 2: • • High activity; chemical biological inertness Photostable; nontoxicity • High recombination; absorption only in UV • Application: • • • Self-cleaning Depollution De-odorizing

Thank you for your attention • This project is funded by the Norwegian Financial Mechanism. Registration number: NF-CZ 07 -ICP-1 -040 -2014. Name of the project: „Formation of research surrounding for young researchers in the field of advanced materials for catalysis and bioapplications“
 Dyduch janusz
Dyduch janusz Eea montpellier
Eea montpellier Eea algorithm
Eea algorithm Eea edmonton
Eea edmonton Eea 600 011
Eea 600 011 Etcte
Etcte Irene del barrio
Irene del barrio Eea
Eea Il viaggio di ulisse scuola primaria
Il viaggio di ulisse scuola primaria Absolute location of oslo
Absolute location of oslo Sweden and norway use equal quantities of resources
Sweden and norway use equal quantities of resources Living and working in norway
Living and working in norway Kinetics and equilibrium
Kinetics and equilibrium Difference between 1st order and zero order kinetics
Difference between 1st order and zero order kinetics Difference between zero and first order kinetics
Difference between zero and first order kinetics Planar kinetics of a rigid body force and acceleration
Planar kinetics of a rigid body force and acceleration Kinetics of a particle: force and acceleration
Kinetics of a particle: force and acceleration Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Myeloblast
Myeloblast Kinematics of rigid bodies problems and solutions
Kinematics of rigid bodies problems and solutions Planar kinetics of a rigid body work and energy
Planar kinetics of a rigid body work and energy Cell kinetics and fermenter design
Cell kinetics and fermenter design Halden prison norway
Halden prison norway Norway water resources
Norway water resources Vikings mc norway
Vikings mc norway Imdi norway
Imdi norway Russefeiring
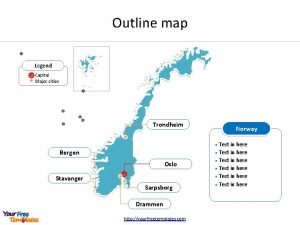
Russefeiring Map of norway with major cities
Map of norway with major cities Norway legal system
Norway legal system Norway religion
Norway religion Religion in norway
Religion in norway Ncis norway
Ncis norway Norway currency
Norway currency Popultion of norway
Popultion of norway Norway ppp
Norway ppp Western norway university of applied sciences
Western norway university of applied sciences Bixter training program
Bixter training program Dt kontakt kjell
Dt kontakt kjell Religion of norway
Religion of norway Homelessness in norway
Homelessness in norway Base jumping in norway
Base jumping in norway Geodata norway
Geodata norway Pretec group as
Pretec group as