Edoxaban versus Warfarin after Surgical Bioprosthetic Valve Implantation
Edoxaban versus Warfarin after Surgical Bioprosthetic Valve Implantation or Valve Repair Geu-Ru Hong, MD, Ph. D on behalf of the ENAVLE trial investigators Division of Cardiology, Severance Cardiovascular Hospital Yonsei University College of Medicine, Seoul, Korea

Disclosure Conflict of Interest Disclosures: There are no conflict of interest disclosures. Funding/Support: This study was supported by a research grant from Daiichi Sankyo. Role of the funder/sponsor: No funder/sponsor had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data.

Backgrounds Early postoperative anticoagulation with warfarin is recommended in patients undergoing surgical bioprosthetic valve implantation or valve repair. However, it is unclear whether direct oral anticoagulant can be an alternative to warfarin in this population.

Objective To compare the efficacy and safety of edoxaban with warfarin for 3 months in patients who underwent surgical bioprosthetic valve implantation or valve repair.
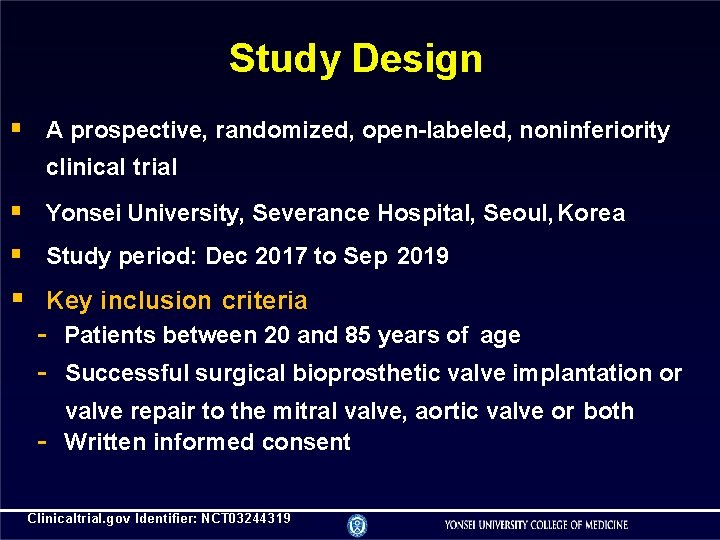
Study Design A prospective, randomized, open-labeled, noninferiority clinical trial Yonsei University, Severance Hospital, Seoul, Korea Study period: Dec 2017 to Sep 2019 Key inclusion criteria - Patients between 20 and 85 years of age - Successful surgical bioprosthetic valve implantation or - valve repair to the mitral valve, aortic valve or both Written informed consent Clinicaltrial. gov Identifier: NCT 03244319
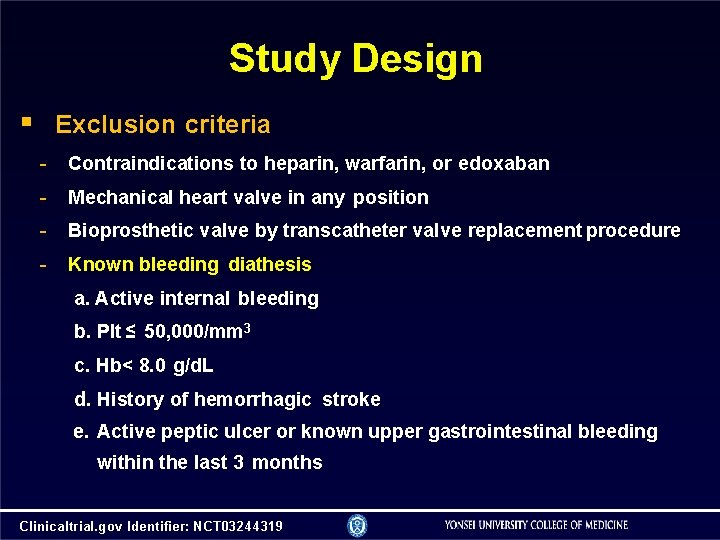
Study Design Exclusion criteria - Contraindications to heparin, warfarin, or edoxaban - Mechanical heart valve in any position - Bioprosthetic valve by transcatheter valve replacement procedure - Known bleeding diathesis a. Active internal bleeding b. Plt ≤ 50, 000/mm 3 c. Hb< 8. 0 g/d. L d. History of hemorrhagic stroke e. Active peptic ulcer or known upper gastrointestinal bleeding within the last 3 months Clinicaltrial. gov Identifier: NCT 03244319
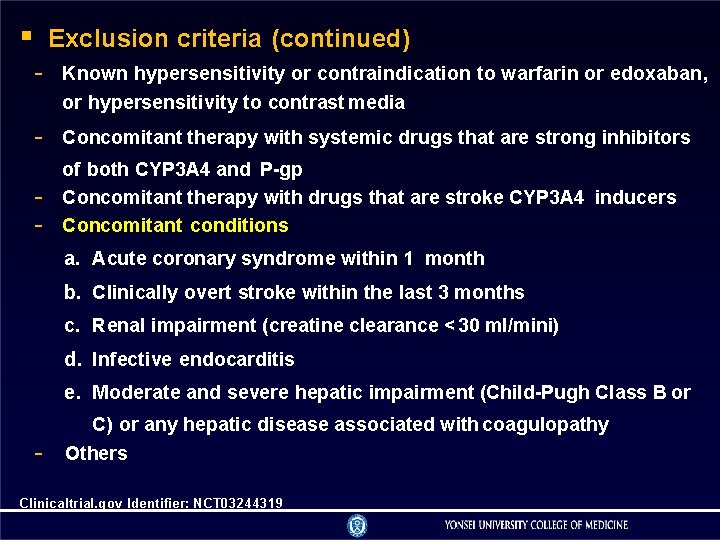
Exclusion criteria (continued) - Known hypersensitivity or contraindication to warfarin or edoxaban, or hypersensitivity to contrast media - Concomitant therapy with systemic drugs that are strong inhibitors - of both CYP 3 A 4 and P-gp Concomitant therapy with drugs that are stroke CYP 3 A 4 inducers Concomitant conditions a. Acute coronary syndrome within 1 month b. Clinically overt stroke within the last 3 months c. Renal impairment (creatine clearance < 30 ml/mini) d. Infective endocarditis e. Moderate and severe hepatic impairment (Child-Pugh Class B or - C) or any hepatic disease associated with coagulopathy Others Clinicaltrial. gov Identifier: NCT 03244319
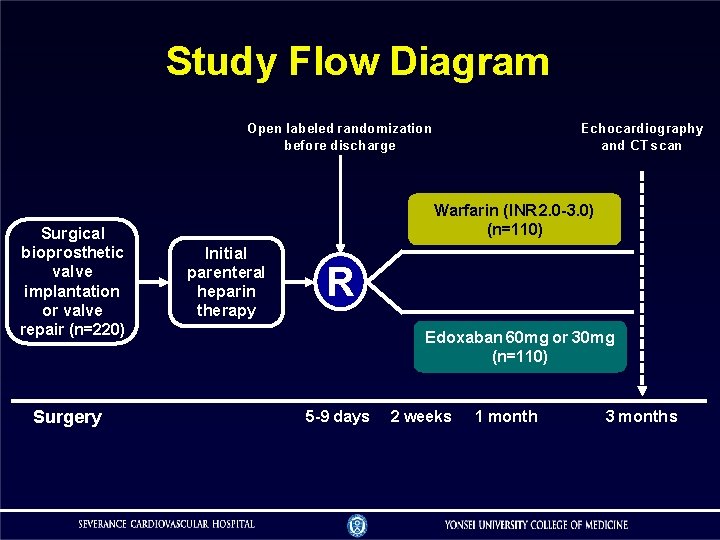
Study Flow Diagram Open labeled randomization before discharge Surgical bioprosthetic valve implantation or valve repair (n=220) Surgery Echocardiography and CT scan Warfarin (INR 2. 0 -3. 0) (n=110) Initial parenteral heparin therapy R Edoxaban 60 mg or 30 mg (n=110) 5 -9 days 2 weeks 1 month 3 months
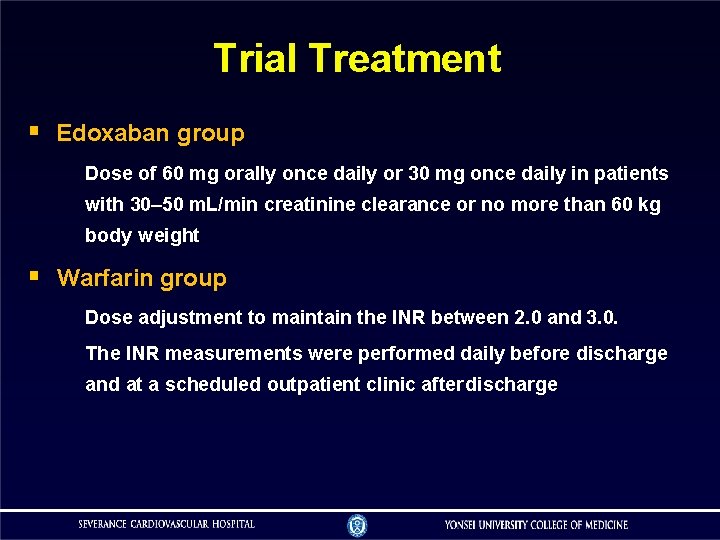
Trial Treatment Edoxaban group Dose of 60 mg orally once daily or 30 mg once daily in patients with 30– 50 m. L/min creatinine clearance or no more than 60 kg body weight Warfarin group Dose adjustment to maintain the INR between 2. 0 and 3. 0. The INR measurements were performed daily before discharge and at a scheduled outpatient clinic after discharge
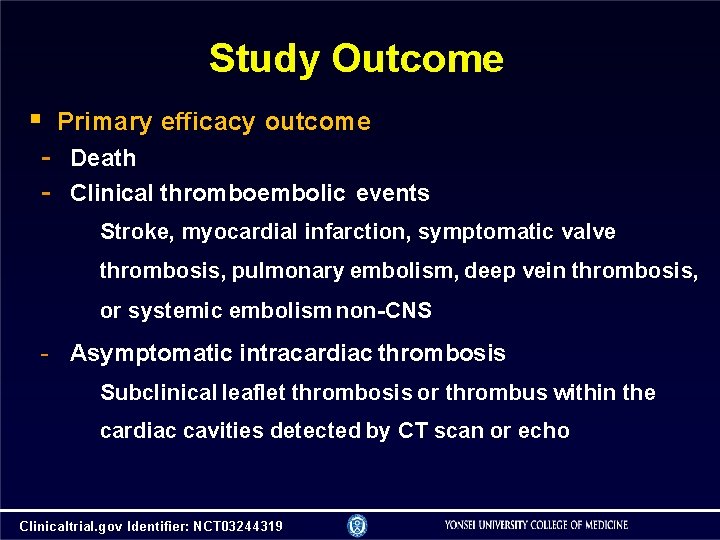
Study Outcome Primary efficacy outcome - Death - Clinical thromboembolic events Stroke, myocardial infarction, symptomatic valve thrombosis, pulmonary embolism, deep vein thrombosis, or systemic embolism non-CNS - Asymptomatic intracardiac thrombosis Subclinical leaflet thrombosis or thrombus within the cardiac cavities detected by CT scan or echo Clinicaltrial. gov Identifier: NCT 03244319
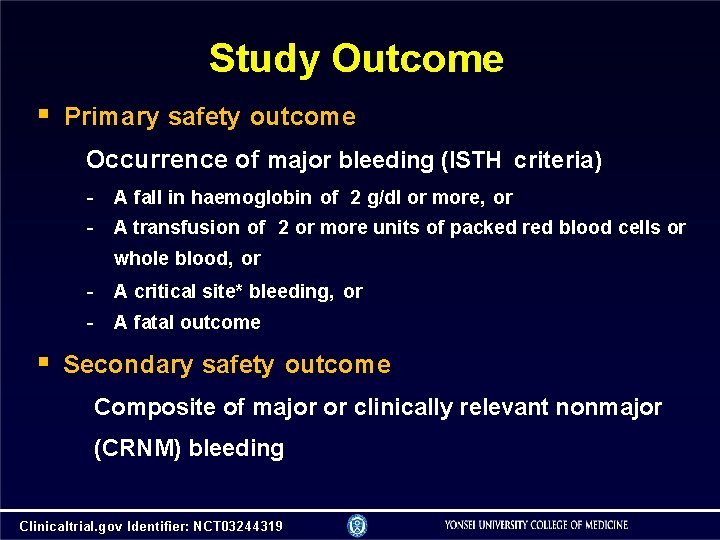
Study Outcome Primary safety outcome Occurrence of major bleeding (ISTH criteria) - A fall in haemoglobin of 2 g/dl or more, or A transfusion of 2 or more units of packed red blood cells or whole blood, or - A critical site* bleeding, or - A fatal outcome Secondary safety outcome Composite of major or clinically relevant nonmajor (CRNM) bleeding Clinicaltrial. gov Identifier: NCT 03244319
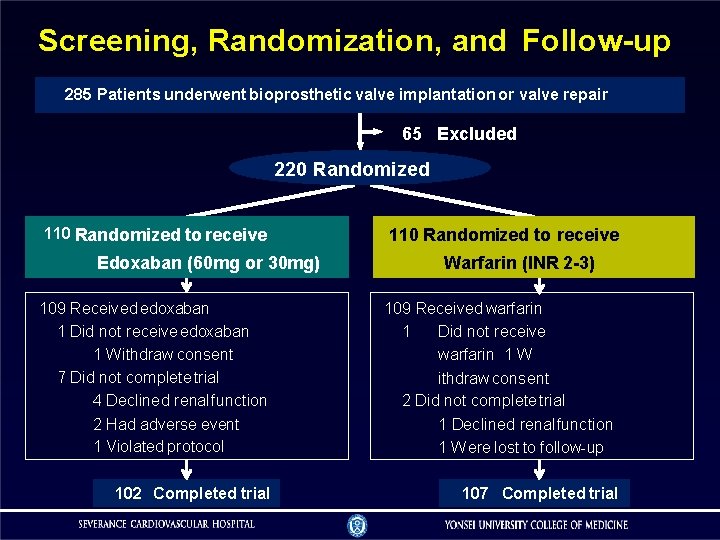
Screening, Randomization, and Follow-up 285 Patients underwent bioprosthetic valve implantation or valve repair 65 Excluded 220 Randomized 110 Randomized to receive Edoxaban (60 mg or 30 mg) 109 Received edoxaban 1 Did not receive edoxaban 1 W ithdraw consent 7 Did not complete trial 4 Declined renal function 2 Had adverse event 1 Violated protocol 102 Completed trial 110 Randomized to receive Warfarin (INR 2 -3) 109 Received warfarin 1 Did not receive warfarin 1 W ithdraw consent 2 Did not complete trial 1 Declined renal function 1 W ere lost to follow-up 107 Completed trial
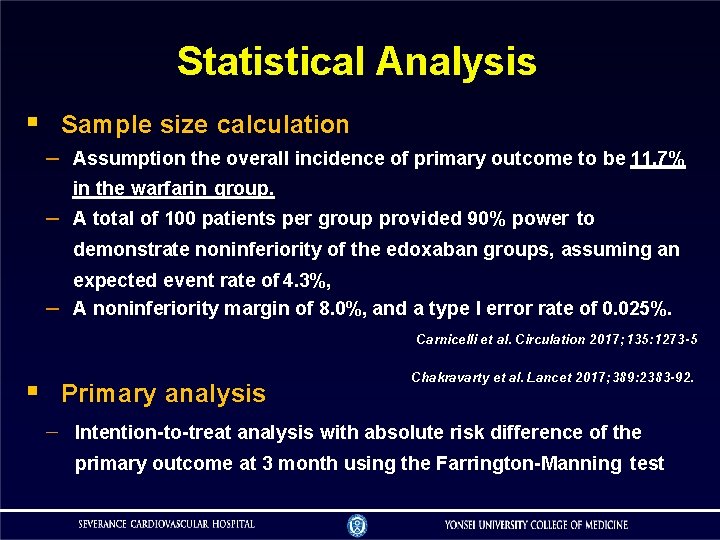
Statistical Analysis Sample size calculation – Assumption the overall incidence of primary outcome to be 11. 7% – in the warfarin group. A total of 100 patients per group provided 90% power to demonstrate noninferiority of the edoxaban groups, assuming an – expected event rate of 4. 3%, A noninferiority margin of 8. 0%, and a type I error rate of 0. 025%. Carnicelli et al. Circulation 2017; 135: 1273 -5 Primary analysis Chakravarty et al. Lancet 2017; 389: 2383 -92. Intention-to-treat analysis with absolute risk difference of the primary outcome at 3 month using the Farrington-Manning test
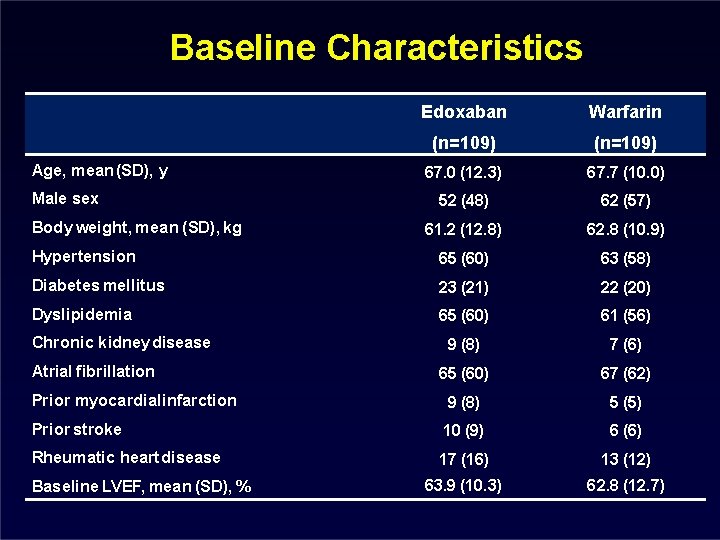
Baseline Characteristics Edoxaban Warfarin (n=109) 67. 0 (12. 3) 67. 7 (10. 0) 52 (48) 62 (57) 61. 2 (12. 8) 62. 8 (10. 9) Hypertension 65 (60) 63 (58) Diabetes mellitus 23 (21) 22 (20) Dyslipidemia 65 (60) 61 (56) 9 (8) 7 (6) 65 (60) 67 (62) Prior myocardial infarction 9 (8) 5 (5) Prior stroke 10 (9) 6 (6) Rheumatic heart disease 17 (16) 13 (12) 63. 9 (10. 3) 62. 8 (12. 7) Age, mean (SD), y Male sex Body weight, mean (SD), kg Chronic kidney disease Atrial fibrillation Baseline LVEF, mean (SD), %
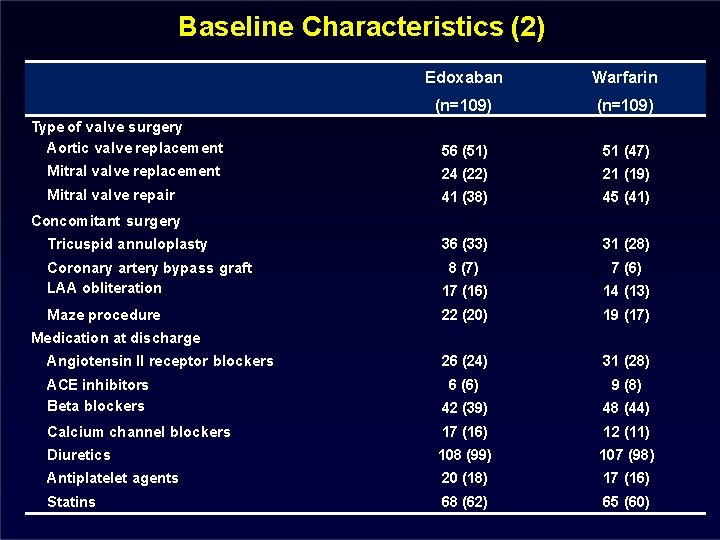
Baseline Characteristics (2) Edoxaban Warfarin (n=109) Type of valve surgery Aortic valve replacement 56 (51) 51 (47) Mitral valve replacement 24 (22) 21 (19) Mitral valve repair 41 (38) 45 (41) 36 (33) 31 (28) Concomitant surgery Tricuspid annuloplasty Coronary artery bypass graft LAA obliteration 8 (7) 7 (6) 17 (16) 14 (13) Maze procedure 22 (20) 19 (17) 26 (24) 31 (28) Medication at discharge Angiotensin II receptor blockers ACE inhibitors Beta blockers 6 (6) 9 (8) 42 (39) 48 (44) Calcium channel blockers 17 (16) 12 (11) Diuretics 108 (99) 107 (98) Antiplatelet agents 20 (18) 17 (16) Statins 68 (62) 65 (60)
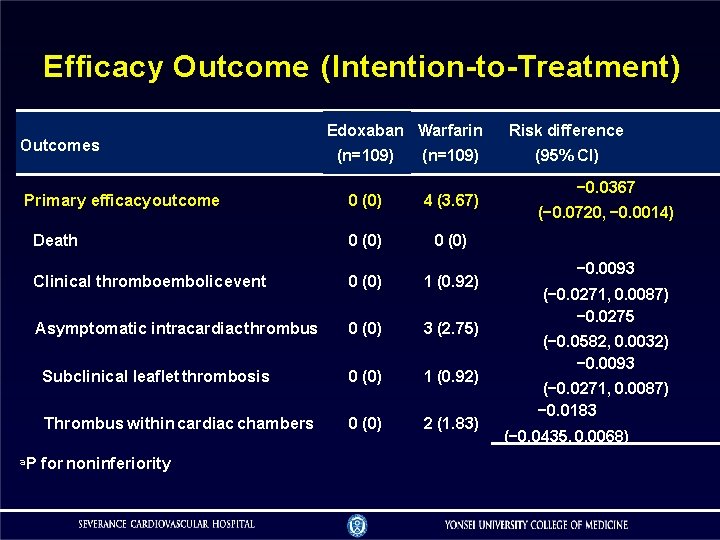
Efficacy Outcome (Intention-to-Treatment) Outcomes Edoxaban Warfarin (n=109) 0 (0) 4 (3. 67) Death 0 (0) Clinical thromboembolic event 0 (0) 1 (0. 92) Asymptomatic intracardiac thrombus 0 (0) 3 (2. 75) Subclinical leaflet thrombosis 0 (0) 1 (0. 92) Thrombus within cardiac chambers 0 (0) 2 (1. 83) Primary efficacy outcome a. P for noninferiority Risk difference (95% CI) − 0. 0367 (− 0. 0720, − 0. 0014) − 0. 0093 (− 0. 0271, 0. 0087) − 0. 0275 (− 0. 0582, 0. 0032) − 0. 0093 (− 0. 0271, 0. 0087) − 0. 0183 (− 0. 0435, 0. 0068)
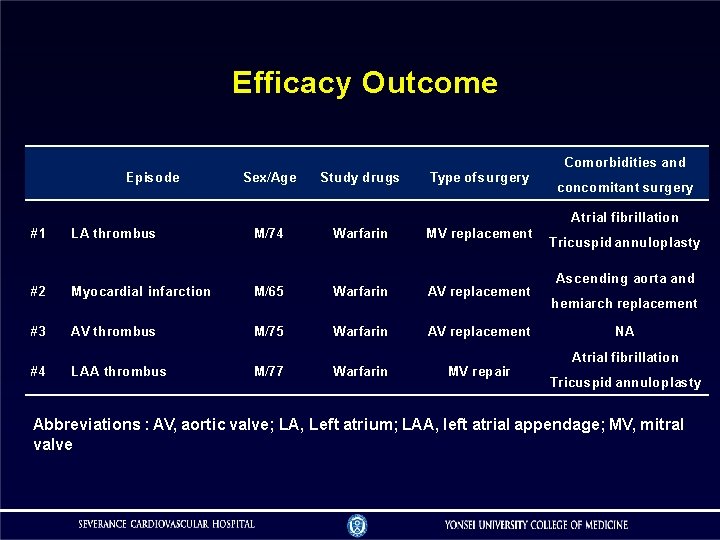
Efficacy Outcome Comorbidities and Episode Sex/Age Study drugs Type of surgery concomitant surgery Atrial fibrillation #1 LA thrombus M/74 Warfarin MV replacement #2 Myocardial infarction M/65 Warfarin AV replacement #3 AV thrombus M/75 Warfarin AV replacement #4 LAA thrombus M/77 Warfarin MV repair Tricuspid annuloplasty Ascending aorta and hemiarch replacement NA Atrial fibrillation Tricuspid annuloplasty Abbreviations : AV, aortic valve; LA, Left atrium; LAA, left atrial appendage; MV, mitral valve
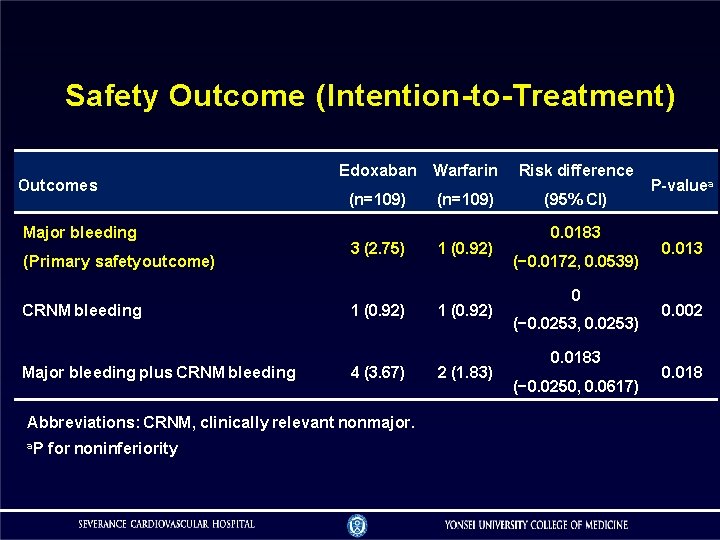
Safety Outcome (Intention-to-Treatment) Outcomes Major bleeding (Primary safety outcome) CRNM bleeding Major bleeding plus CRNM bleeding Edoxaban Warfarin Risk difference (n=109) (95% CI) 3 (2. 75) 1 (0. 92) 4 (3. 67) Abbreviations: CRNM, clinically relevant nonmajor. a. P for noninferiority 2 (1. 83) 0. 0183 (− 0. 0172, 0. 0539) 0 (− 0. 0253, 0. 0253) 0. 0183 (− 0. 0250, 0. 0617) P-valuea 0. 013 0. 002 0. 018
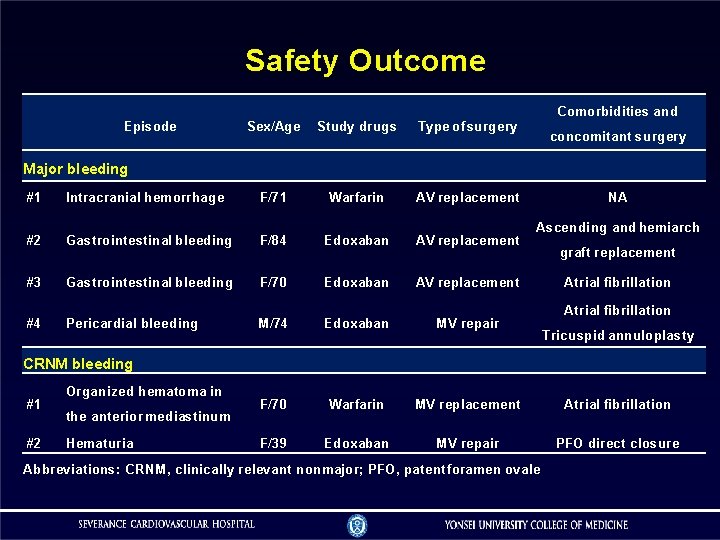
Safety Outcome Comorbidities and Episode Sex/Age Study drugs Type of surgery F/71 Warfarin AV replacement concomitant surgery Major bleeding #1 Intracranial hemorrhage #2 Gastrointestinal bleeding F/84 Edoxaban AV replacement #3 Gastrointestinal bleeding F/70 Edoxaban AV replacement #4 Pericardial bleeding NA Ascending and hemiarch graft replacement Atrial fibrillation M/74 Edoxaban MV repair F/70 Warfarin MV replacement Atrial fibrillation F/39 Edoxaban MV repair PFO direct closure Tricuspid annuloplasty CRNM bleeding #1 #2 Organized hematoma in the anterior mediastinum Hematuria Abbreviations: CRNM, clinically relevant nonmajor; PFO, patent foramen ovale
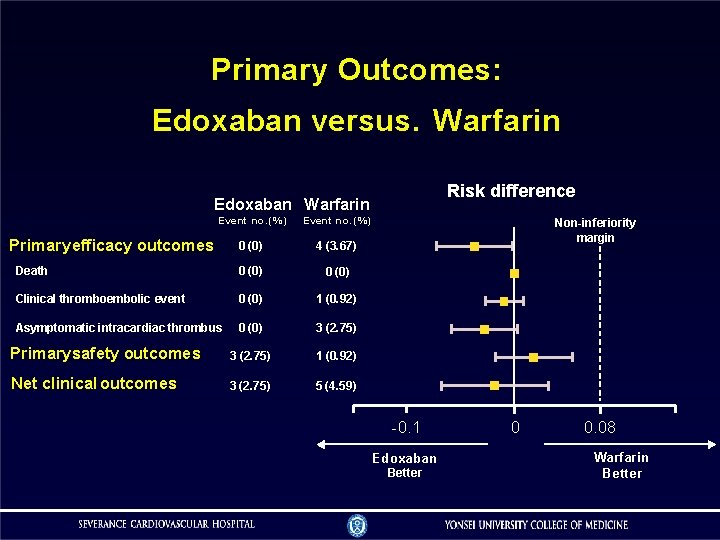
Primary Outcomes: Edoxaban versus. Warfarin Risk difference Edoxaban Warfarin Event no. (%) 0 (0) 4 (3. 67) Death 0 (0) Clinical thromboembolic event 0 (0) 1 (0. 92) Asymptomatic intracardiac thrombus 0 (0) 3 (2. 75) Primary safety outcomes 3 (2. 75) 1 (0. 92) Net clinical outcomes 3 (2. 75) 5 (4. 59) Primary efficacy outcomes Non-inferiority margin -0. 1 Edoxaban Better 0 0. 08 Warfarin Better
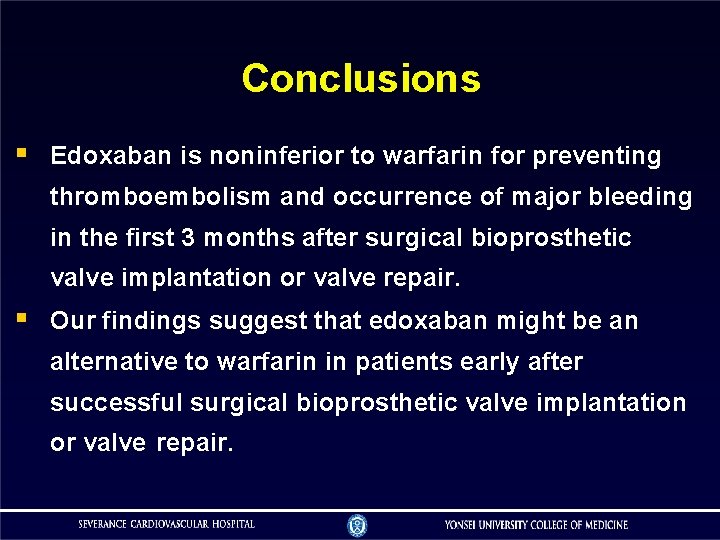
Conclusions Edoxaban is noninferior to warfarin for preventing thromboembolism and occurrence of major bleeding in the first 3 months after surgical bioprosthetic valve implantation or valve repair. Our findings suggest that edoxaban might be an alternative to warfarin in patients early after successful surgical bioprosthetic valve implantation or valve repair.
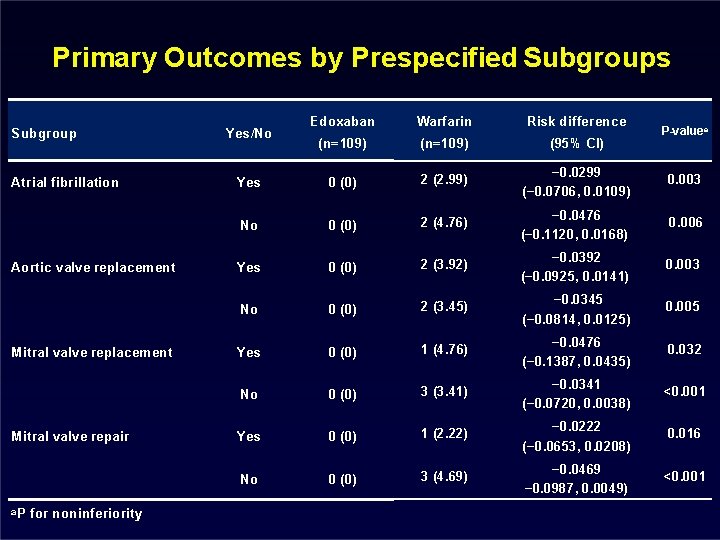
Primary Outcomes by Prespecified Subgroups Subgroup Atrial fibrillation Aortic valve replacement Mitral valve repair a. P for noninferiority Edoxaban Warfarin Risk difference (n=109) (95% CI) Yes 0 (0) 2 (2. 99) − 0. 0299 (− 0. 0706, 0. 0109) 0. 003 No 0 (0) 2 (4. 76) − 0. 0476 (− 0. 1120, 0. 0168) 0. 006 Yes 0 (0) 2 (3. 92) − 0. 0392 (− 0. 0925, 0. 0141) 0. 003 No 0 (0) 2 (3. 45) − 0. 0345 (− 0. 0814, 0. 0125) 0. 005 Yes 0 (0) 1 (4. 76) − 0. 0476 (− 0. 1387, 0. 0435) 0. 032 No 0 (0) 3 (3. 41) − 0. 0341 (− 0. 0720, 0. 0038) <0. 001 Yes 0 (0) 1 (2. 22) − 0. 0222 (− 0. 0653, 0. 0208) 0. 016 No 0 (0) 3 (4. 69) − 0. 0469 − 0. 0987, 0. 0049) <0. 001 Yes/No P-valuea
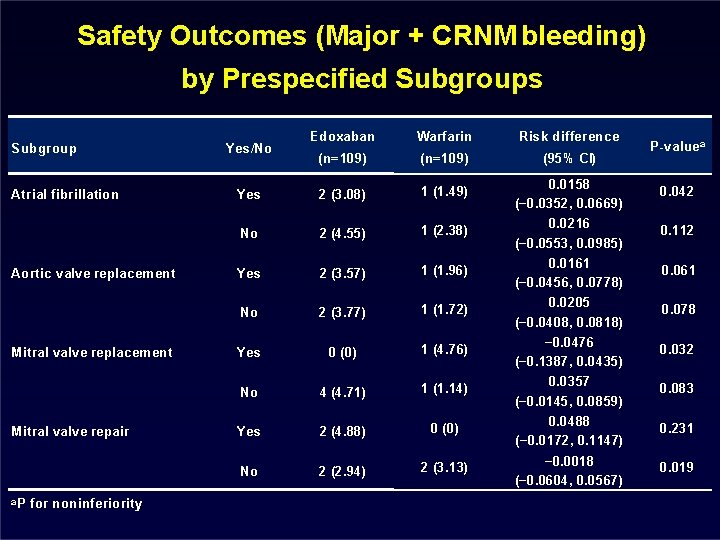
Safety Outcomes (Major + CRNM bleeding) by Prespecified Subgroups Subgroup Atrial fibrillation Aortic valve replacement Mitral valve repair a. P for noninferiority Edoxaban Warfarin Risk difference (n=109) (95% CI) Yes 2 (3. 08) 1 (1. 49) 0. 0158 (− 0. 0352, 0. 0669) 0. 042 No 2 (4. 55) 1 (2. 38) 0. 0216 (− 0. 0553, 0. 0985) 0. 112 Yes 2 (3. 57) 1 (1. 96) 0. 0161 (− 0. 0456, 0. 0778) 0. 061 No 2 (3. 77) 1 (1. 72) 0. 0205 (− 0. 0408, 0. 0818) 0. 078 Yes 0 (0) 1 (4. 76) − 0. 0476 (− 0. 1387, 0. 0435) 0. 032 No 4 (4. 71) 1 (1. 14) 0. 0357 (− 0. 0145, 0. 0859) 0. 083 Yes 2 (4. 88) 0 (0) 0. 0488 (− 0. 0172, 0. 1147) 0. 231 No 2 (2. 94) 2 (3. 13) − 0. 0018 (− 0. 0604, 0. 0567) 0. 019 Yes/No P-valuea
- Slides: 23