Earths Atmosphere Atmosphere Envelope of gases that surround

























- Slides: 34

Earth’s Atmosphere

Atmosphere Envelope of gases that surround the Earth n Protects the Earth n Provides materials necessary to support all forms of life n

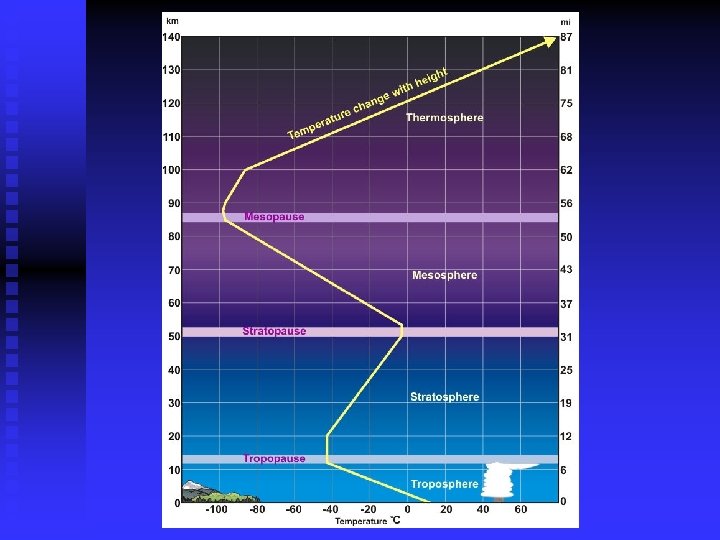
4 Regions based on Temperature Troposphere n Stratosphere n Mesosphere n Thermosphere n

Troposphere 0 -12 km n Temperature decreases with altitude u Minimum of 215 K n Weather n Upper limit is the tropopause n

Stratosphere 10 -50 km n Temperature increases with altitude u Maximum of 275 K n Upper limit is the stratopause n

Mesosphere 50 -85 km n Temperature decreases with altitude u Minimum of 190 K n Upper limit is the mesopause n

Thermosphere Above 85 km n Temperature increases with altitude n


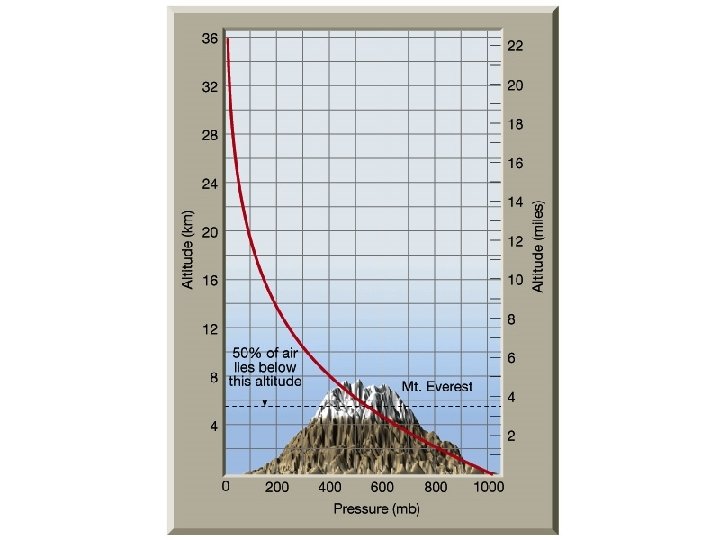
Pressure n Decreases in a regular way with increasing elevation n Troposphere and stratosphere account for 99. 9% of the mass of the atmosphere


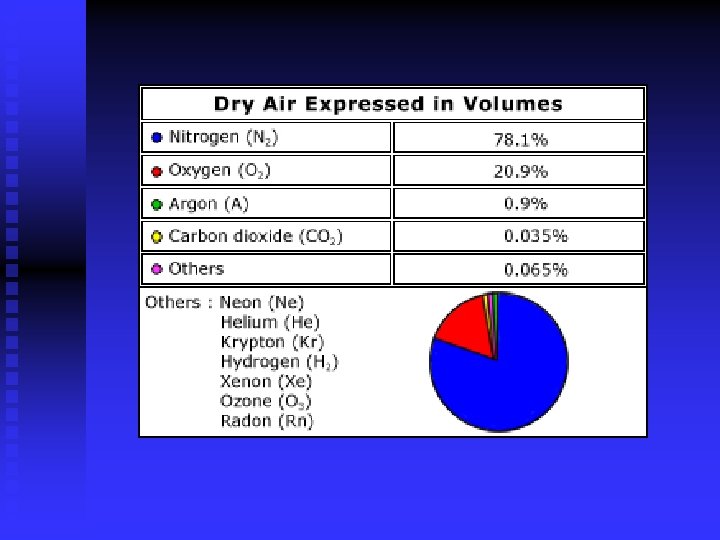
Earth’s Composition N 2 and O 2 make up 99% of atmosphere n CO 2 n Noble gases n TABLE 18. 1 n


Parts per Million Unit of concentration n One part by volume in 1 million volume units of the whole n Volume is proportionate to mole u Volume fraction = mole fraction n

N 2 versus O 2 n N 2 has a triple bond and O 2 has a double bond u O 2 much more reactive and has lower bond energy than N 2

Outer Regions of the Atmosphere n Beyond stratosphere n Outer defense against radiation and highenergy particles

Photodissociation n Shorter-wavelength/ higher-energy radiations in the ultraviolet range of spectrum cause chemical changes n For radiation to fall on Earth’s atmosphere: u Photons with sufficient energy u Molecules absorb photons

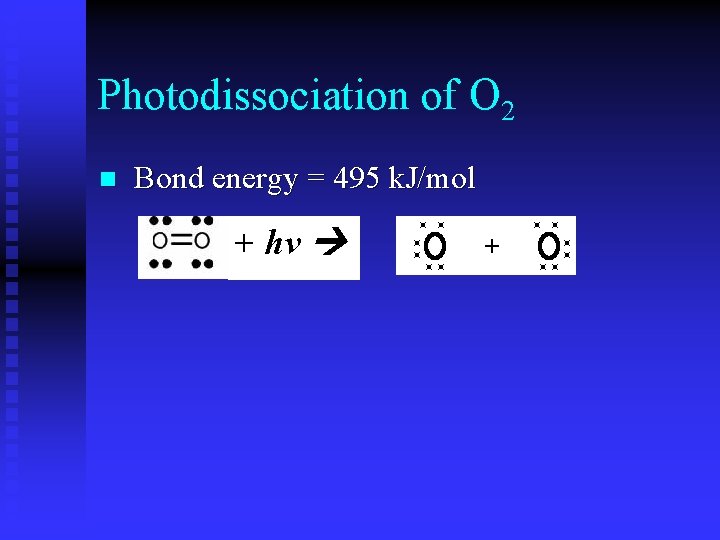
Photodissociation Rupture of a chemical bond resulting from absorption of a photon by a molecule n No ions formed n ½ of electrons stay with one of the atoms & ½ stay with the other u 2 neutral particles n

Photodissociation of O 2 n Bond energy = 495 k. J/mol + hv +

Photoionization n Occurs when a molecule absorbs radiation (a photon) and the absorbed energy causes an electron to be ejected from the molecule u Becomes positively charged ion

Ozone in the Upper Atmosphere n O 3 is the key absorber of photons having wavelengths from 240 -310 nm u Below altitude of 90 km, most shortwavelengths (< 240 nm) have been absorbed by N 2, O 2, and atomic O

Continued n 30 -90 km: + O 2 O 3* *excess energy (releases 105 k. J/mol)

Continued n O 3 collides with other atoms or molecules, M (usually N 2 or O 2), & transfers energy O + O 2 O 3* + M O 3 + M* O + O 2 + M O 3 + M*

Effects on Rate of O 3 Formation 1. Presence of O atoms (favored at higher altitudes) 2. Molecular collisions (favored at lower altitudes)

Continued n Highest rate of O 3 formation occurs in a band at 50 km altitude n 90% of O 3 is found in the stratosphere

After Formation n O 3 does not last long n It absorbs solar radiation and decomposes back into O and O 2

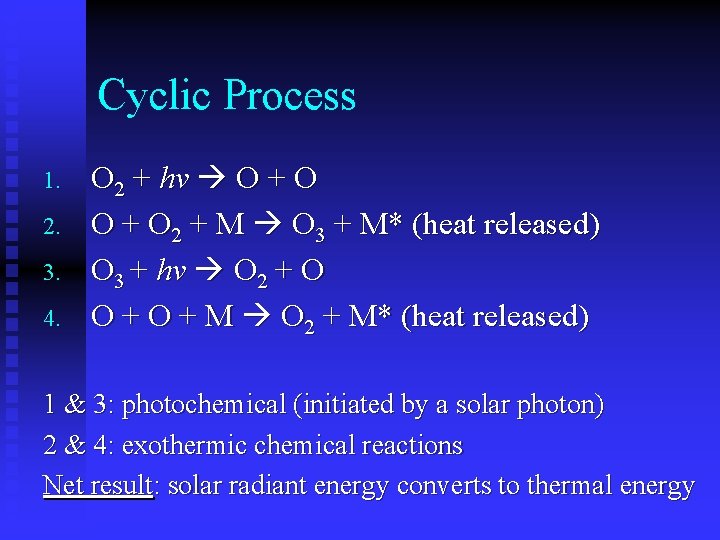
Cyclic Process 1. 2. 3. 4. O 2 + hv O + O 2 + M O 3 + M* (heat released) O 3 + hv O 2 + O O + M O 2 + M* (heat released) 1 & 3: photochemical (initiated by a solar photon) 2 & 4: exothermic chemical reactions Net result: solar radiant energy converts to thermal energy

Depletion of O 3 Layer n 1970 s: CFCs depleting ozone CF Cl 3 and CF 2 Cl 2 u Used in refrigerators, propellants, foaming agents u n CFCs diffuse in stratosphere u Exposed to radiation t Photodissociation occurs

Photodissociation of CFCs C-Cl bond is weaker than C-F bond n Free Cl atoms are formed when = 190 -225 nm u Greatest at altitude of 30 km n CF 2 Cl 2 + hv CF 2 Cl + Cl

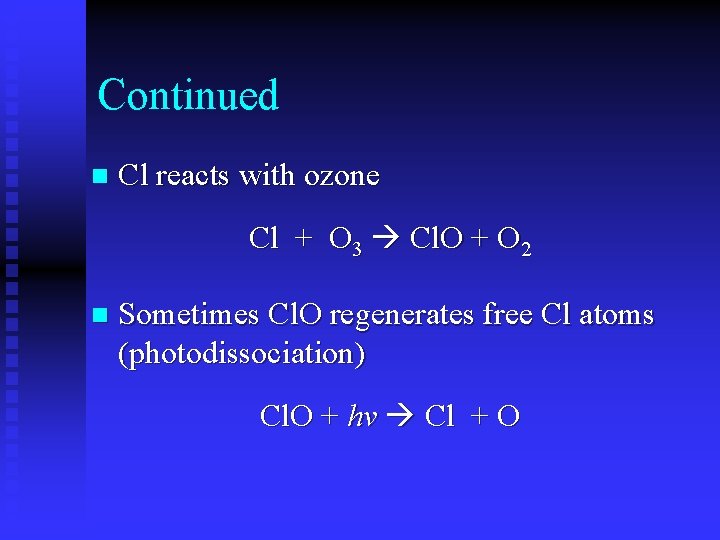
Continued n Cl reacts with ozone Cl + O 3 Cl. O + O 2 n Sometimes Cl. O regenerates free Cl atoms (photodissociation) Cl. O + hv Cl + O

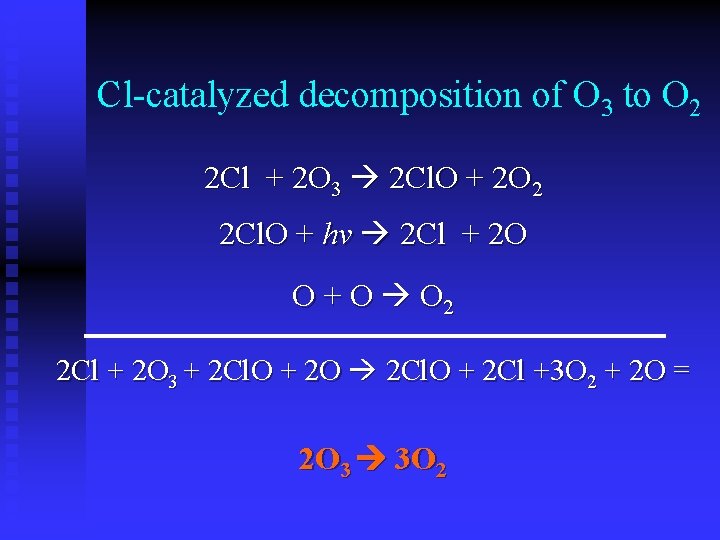
Cl-catalyzed decomposition of O 3 to O 2 2 Cl + 2 O 3 2 Cl. O + 2 O 2 2 Cl. O + hv 2 Cl + 2 O O + O O 2 2 Cl + 2 O 3 + 2 Cl. O + 2 O 2 Cl. O + 2 Cl +3 O 2 + 2 O = 2 O 3 3 O 2

Limiting use of CFCs 1987 Montreal Protocol on Substances that Deplete the Ozone Layer n 1992: 100 nations agreed to ban CFC production by 1996 n

Replacing CFCs n Hydrofluorocarbons u C-H bond replaces that of C-Cl t ex: CH 2 FCF 3 (HFC-134 a)

Natural Depletion Natural sources that contribute Cl and Br to atmosphere (the methyl's) u CH 3 Cl and CH 3 Br n 1/3 of depletion (2/3 human activities) n

Homework n Page 797 u 15 -21 odd only