Earth Systems 3209 Earth Science 1000 MUN Midterm





































































- Slides: 135

Earth Systems 3209/ Earth Science 1000 (MUN) Midterm Review

What is Earth Science? ? • Earth Science differs from other sciences in that: 1. Earth Science has a global perspective 2. Earth Science draws from all other areas of science 3. Earth Science requires a consideration of vast amounts of time

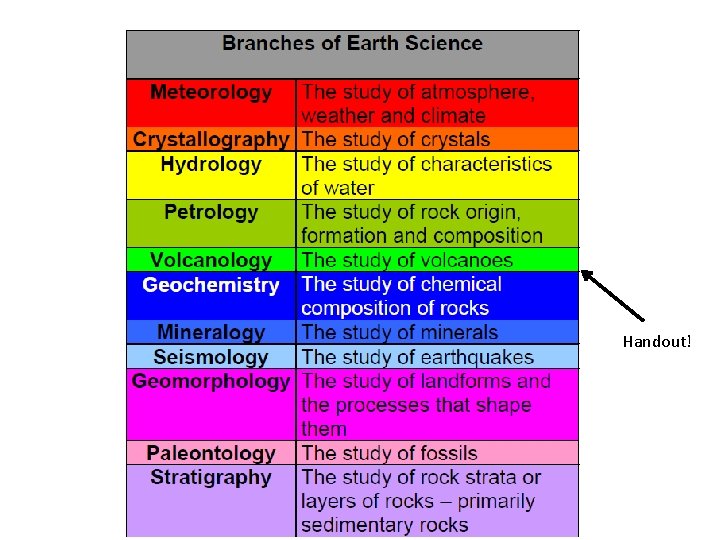
4 Major Branches of Earth Science • Geology: examines earth, its form and composition, and the changes it has undergone and is undergoing. • Meteorology: study of the atmosphere and atmospheric phenomena; weather and climate. • Astronomy: study of the universe; it includes the observation and interpretation of celestial bodies and phenomena. • Oceanography: study of the oceans and oceanic phenomena.

Handout!

Evidence, Theories, Paradigms Scientific Knowledge • Evidence – facts collected by observation and measurement which serve as a springboard for development of theories • Hypothesis: Is a tentative or untested explanation to explain how or why things happen in an observed manner. • Theory: A well tested and widely accepted view that explains certain observable facts. • Paradigm: Is a theory that is held with a high degree of confidence with a lot of

Formation of the Universe • Creationism (considered a Non-Scientific View): • Belief in the bible, other holy texts or spoken word that led to the idea that Earth was created by a creator with purpose

Big Bang Theory • Proposes that universe originated as a single mass that exploded around 15 billion years ago. (best estimate is 13. 7 bya) • After a few billion years material cooled and condensed into stars and galaxies. • The explosion caused continuous expansion so galaxies moved away from one another. • About 5 bya our solar system formed within the Milky Way galaxy • Expansion and cooling continues.

Solar Nebular Hypothesis pg. 19, 628 • Explains the formation of the solar system which began about 5 bya (at least 10 by after the Big Bang occurred)

4 stages of Solar Nebular Hypothesis 1. Nebula Huge rotating cloud of dust and gas contracts due to gravity to form a “nebula”

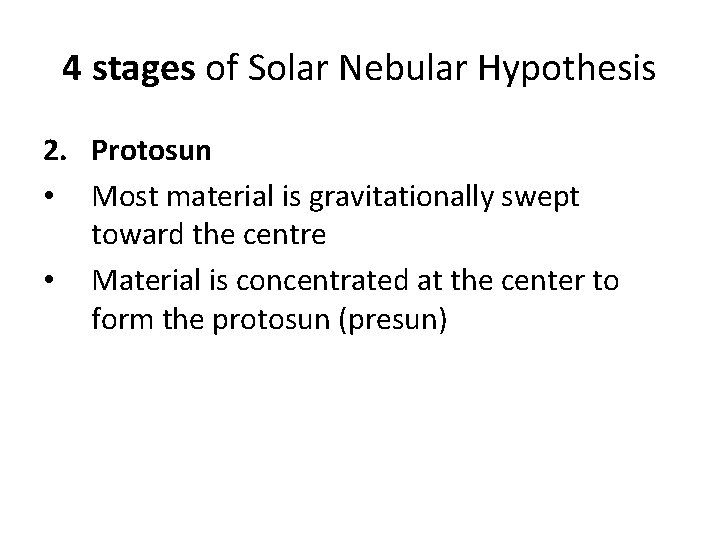
4 stages of Solar Nebular Hypothesis 2. Protosun • Most material is gravitationally swept toward the centre • Material is concentrated at the center to form the protosun (presun)

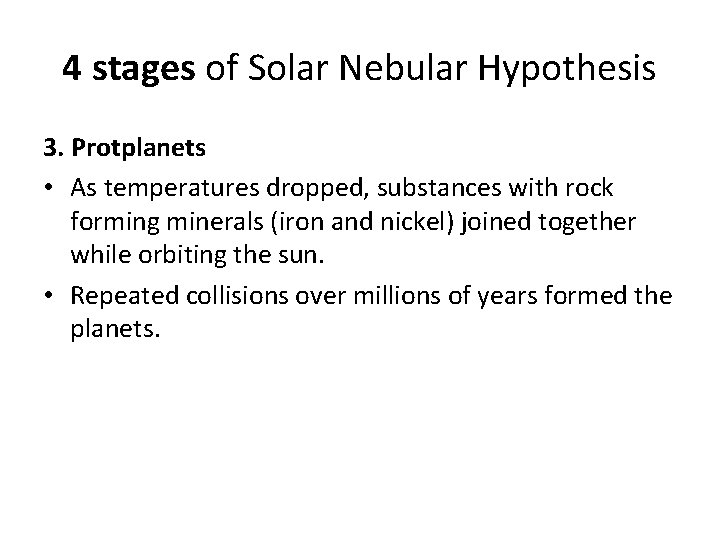
4 stages of Solar Nebular Hypothesis 3. Protplanets • As temperatures dropped, substances with rock forming minerals (iron and nickel) joined together while orbiting the sun. • Repeated collisions over millions of years formed the planets.

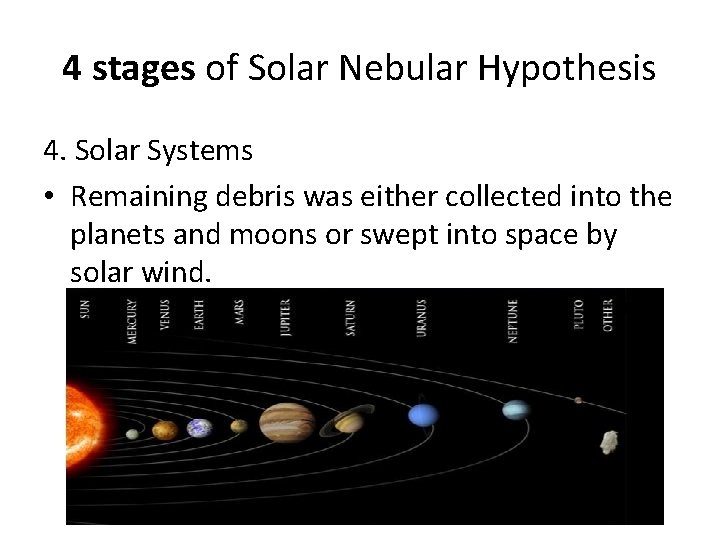
4 stages of Solar Nebular Hypothesis 4. Solar Systems • Remaining debris was either collected into the planets and moons or swept into space by solar wind.

STSE • Remember: • Universe Vs. Solar System • The Universe: incorporates several solar systems. • A solar system: is represented by planets orbiting stars. • Habitable Zone (Goldilocks zone) • This zone in a solar system is where liquid water can exist on the surface and not too far from the star it is orbiting (too cold) or too close to the start it is orbiting (too hot). Just right!

STSE • If you can find planets orbiting stars (other than the sun), then you have found a solar system. • There have been 1500 solar systems found to date. • 1. 2. 3. 4. 5. Five Methods for Finding Solar Systems Radial Velocity Method Transit Photometry Method Astrometry Method Microlensing Method Direct Imaging

STSE • Transit: The passage of a planet between a star and the Earth. • Extrasolar planets: Planets that exist in other solar systems (as opposed to the solar system where Earth exists). These are called exoplanets.

STSE • The Solar Nebula Hypothesis infers that a solar system should have rocky inner planets and larger gaseous planets much further out. • Many solar systems found do not fit the pattern described above. • Maybe gravity and friction has caused the larger planets to move. • Alone, the “Solar Nebula Hypothesis” seems too simplistic. Maybe it just represents the formation and configuration of early planets.

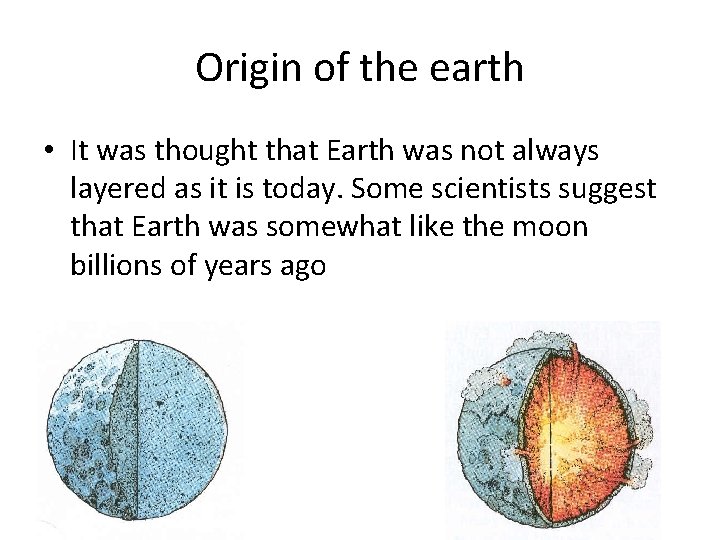
Origin of the earth • It was thought that Earth was not always layered as it is today. Some scientists suggest that Earth was somewhat like the moon billions of years ago

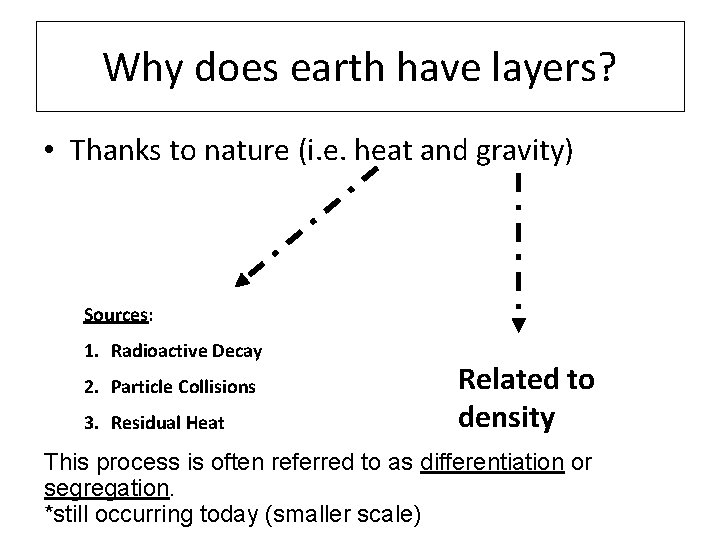
Why does earth have layers? • Thanks to nature (i. e. heat and gravity) Sources: 1. Radioactive Decay 2. Particle Collisions 3. Residual Heat Related to density This process is often referred to as differentiation or segregation. *still occurring today (smaller scale)


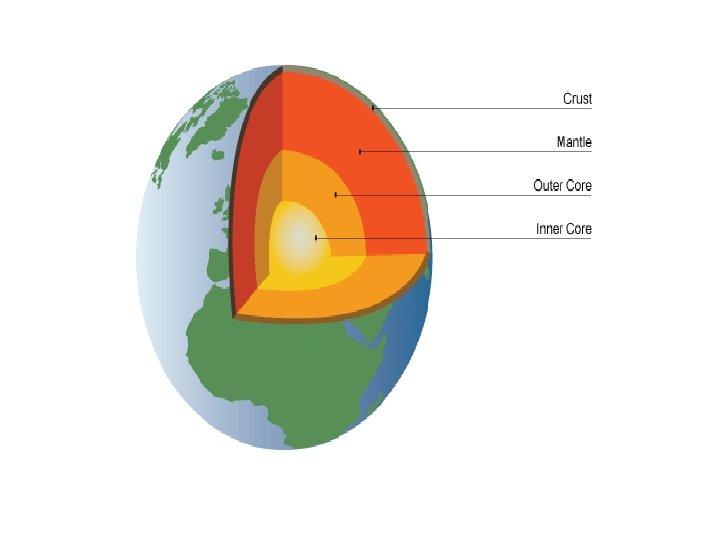
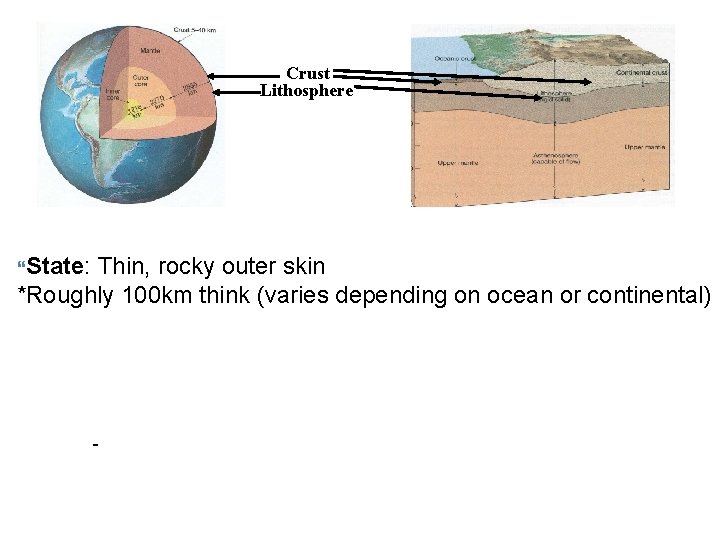
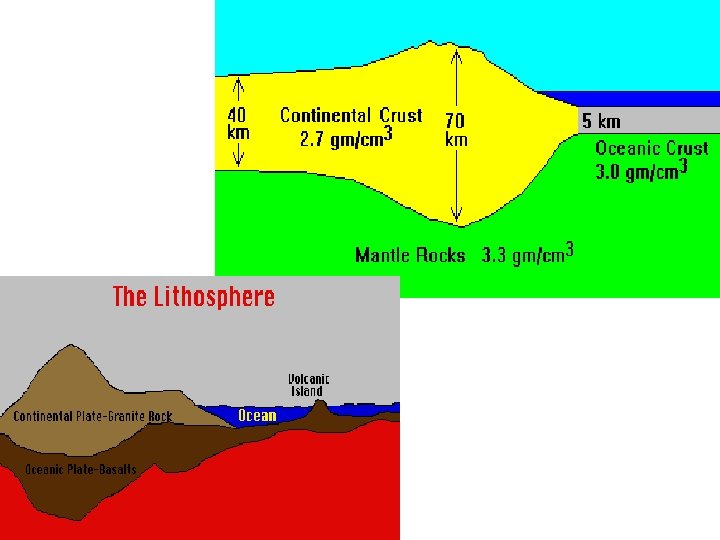
Crust Lithosphere State: Thin, rocky outer skin *Roughly 100 km think (varies depending on ocean or continental) -


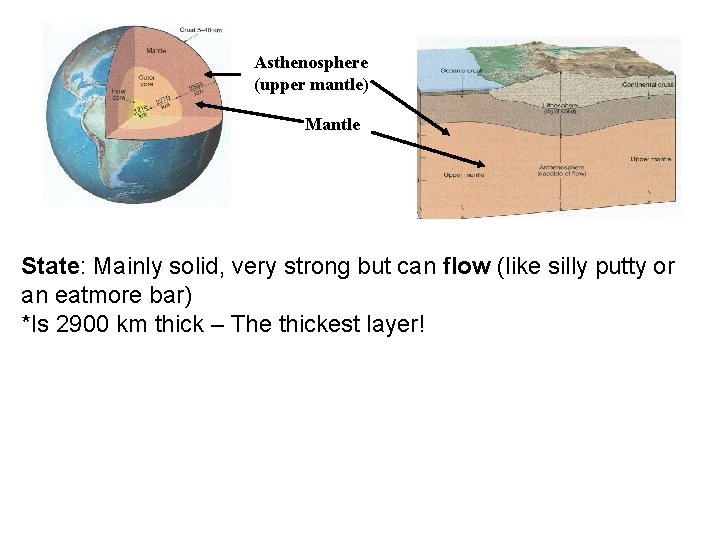
Asthenosphere (upper mantle) Mantle State: Mainly solid, very strong but can flow (like silly putty or an eatmore bar) *Is 2900 km thick – The thickest layer!

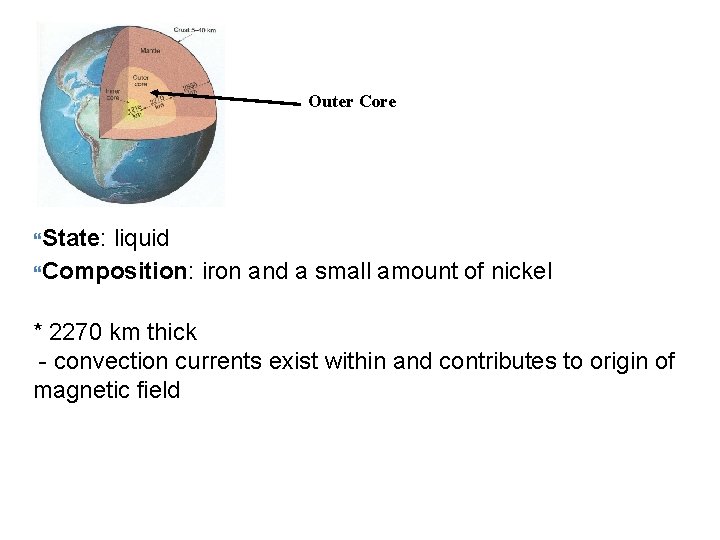
Outer Core State: liquid Composition: iron and a small amount of nickel * 2270 km thick - convection currents exist within and contributes to origin of magnetic field

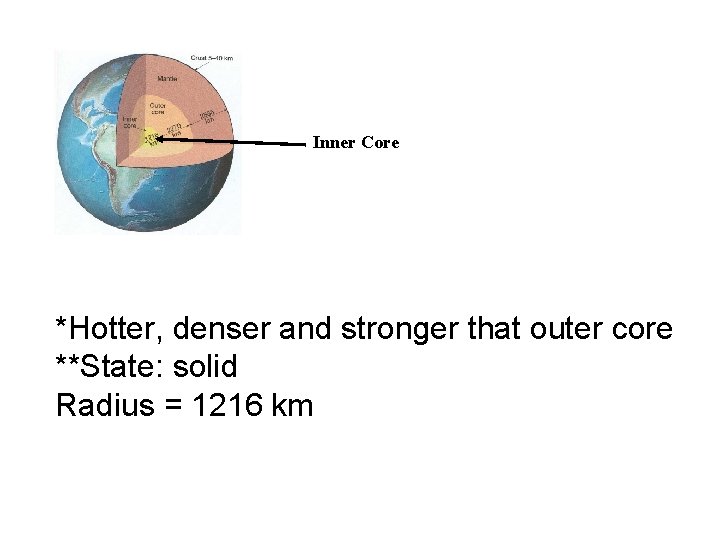
Inner Core *Hotter, denser and stronger that outer core **State: solid Radius = 1216 km

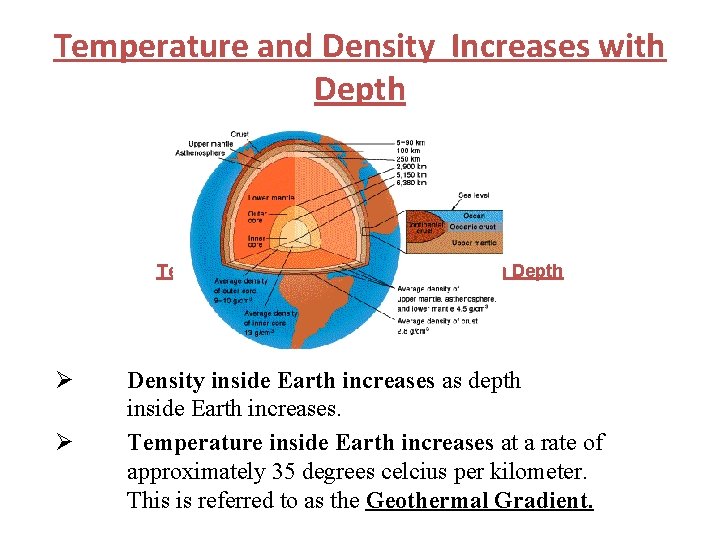
Temperature and Density Increases with Depth Ø Ø Density inside Earth increases as depth inside Earth increases. Temperature inside Earth increases at a rate of approximately 35 degrees celcius per kilometer. This is referred to as the Geothermal Gradient.

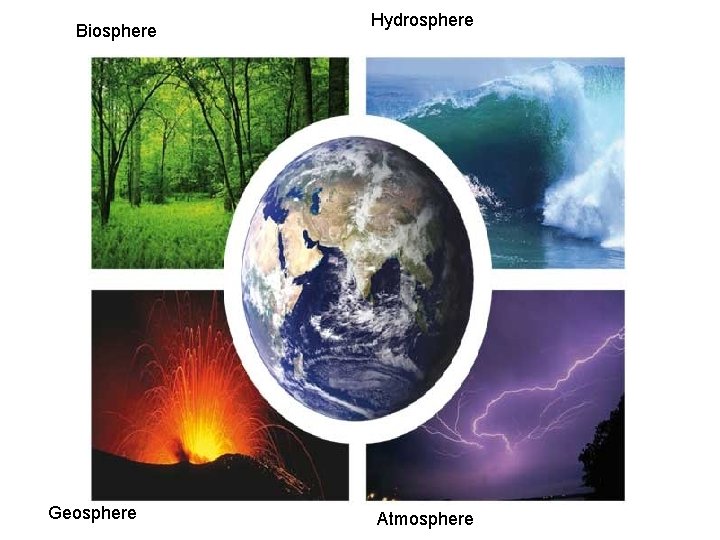
Biosphere Geosphere Hydrosphere Atmosphere

Order of development • Geosphere hydrosphere atmosphere biosphere • Hydrosphere and atmosphere formed from early “outgassing”, where molten material cooled and released dissolved gases into the atmosphere (N 2, H 2 O vapor, CO 2, Ar)

The earth is a complex set of interacting systems • Each sphere influences one another and effects our daily lives

All 4 Spheres • Shoreline

Catastrophism • Is a concept popular in the 1700 -1800’s which states that earth’s landscapes had been shaped primarily by great disasters or catastrophes (floods, storms, earthquakes, volcanic eruptions etc. ) • James Ussher: Anglican Arch Bishop and scholar attempted to fit the rate of change of Earth processes into a relatively young aged Earth. ** Unknown causes which were fast

James Hutton • Scottish Geologist after years of studying landforms and rocks • “ the present is the key to the past” and • “that, the physical, chemical, biological laws that operate today to shape Earth also operated in the past. ”

Uniformitarianism • two key concepts; 1) the geologic processes at work today were also active in the past. 2) the present physical features of Earth were formed by these same processes, at work • over long periods of time.

Absolute time • Identifies the actual date of an event, & pinpoints the exact time in history when something took place. • For example, the extinction of the dinosaurs about 66 million years ago and the age of • Earth is approximately 4. 6 Billion years.

. Relative time • Attempts to place events in a sequence of formation, but does not identify their actual date of occurrence. • Comparing events to each other often does this. • Can’t tell us how long ago something happened; only that it followed one event and preceded another.


relative dating 6 Major Types: 1. 2. 3. 4. 5. 6. Superposition Horizontality Cross-cutting relations Inclusions Unconformities Fossils

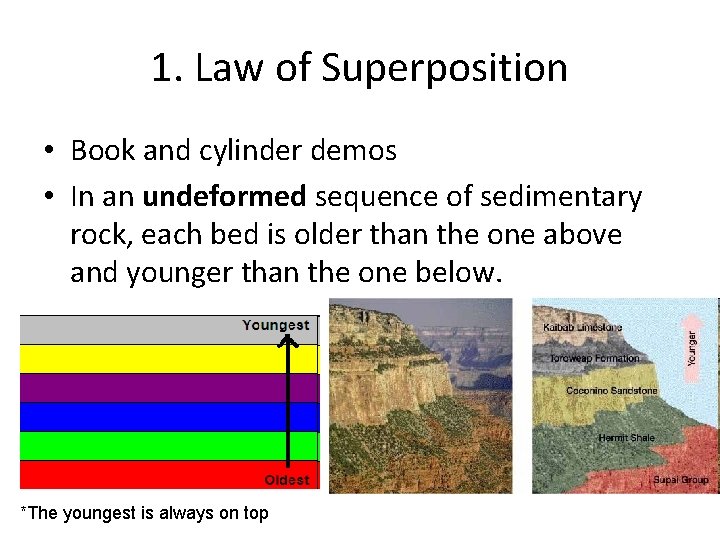
1. Law of Superposition • Book and cylinder demos • In an undeformed sequence of sedimentary rock, each bed is older than the one above and younger than the one below. *The youngest is always on top

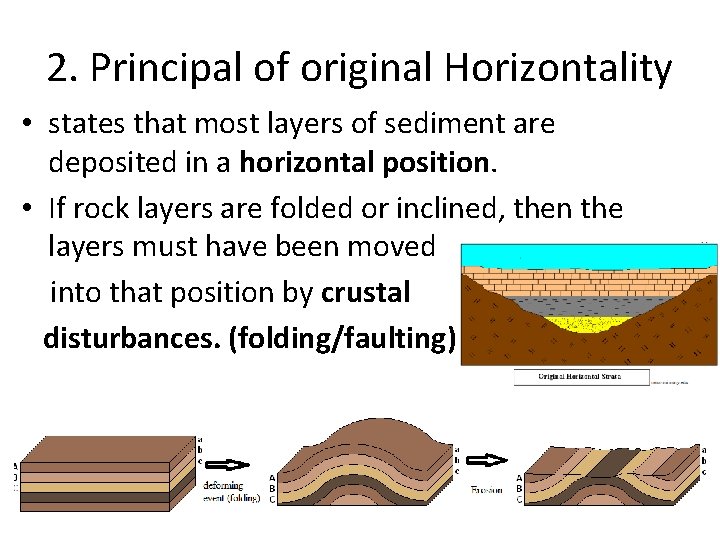
2. Principal of original Horizontality • states that most layers of sediment are deposited in a horizontal position. • If rock layers are folded or inclined, then the layers must have been moved into that position by crustal disturbances. (folding/faulting)

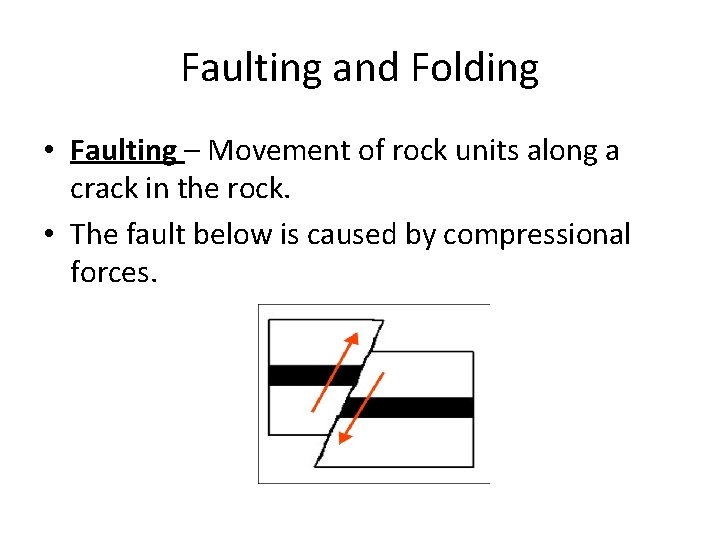
Faulting and Folding • Faulting – Movement of rock units along a crack in the rock. • The fault below is caused by compressional forces.

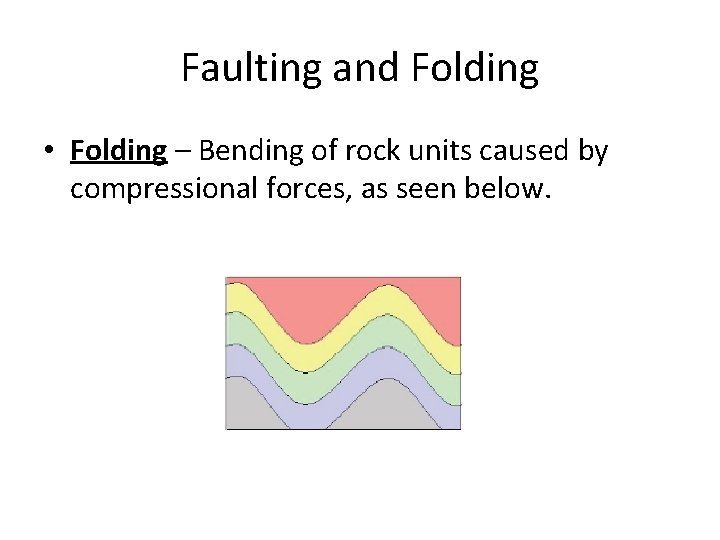
Faulting and Folding • Folding – Bending of rock units caused by compressional forces, as seen below.

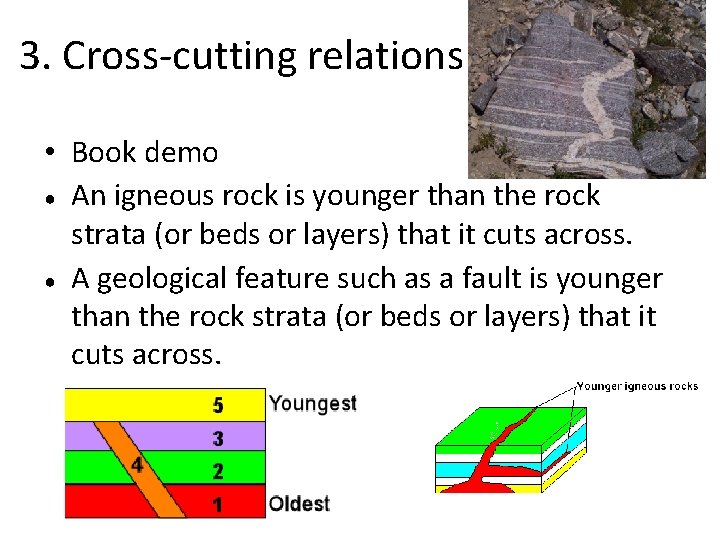
3. Cross-cutting relations • Book demo ● An igneous rock is younger than the rock strata (or beds or layers) that it cuts across. ● A geological feature such as a fault is younger than the rock strata (or beds or layers) that it cuts across.

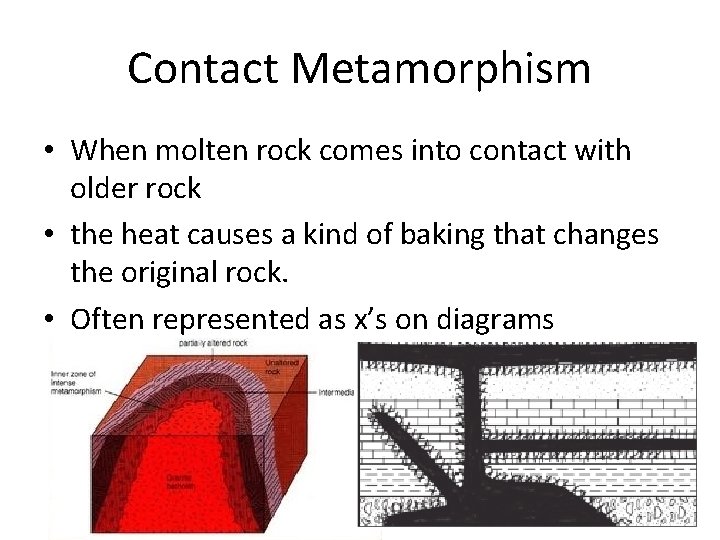
Contact Metamorphism • When molten rock comes into contact with older rock • the heat causes a kind of baking that changes the original rock. • Often represented as x’s on diagrams

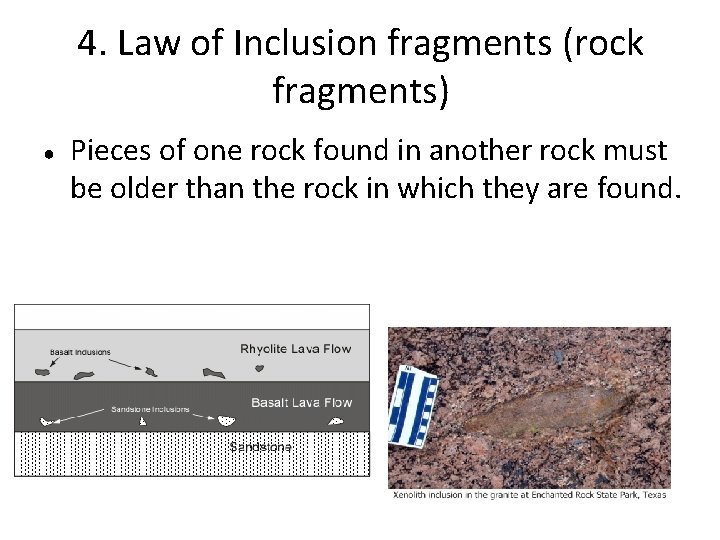
4. Law of Inclusion fragments (rock fragments) ● Pieces of one rock found in another rock must be older than the rock in which they are found.

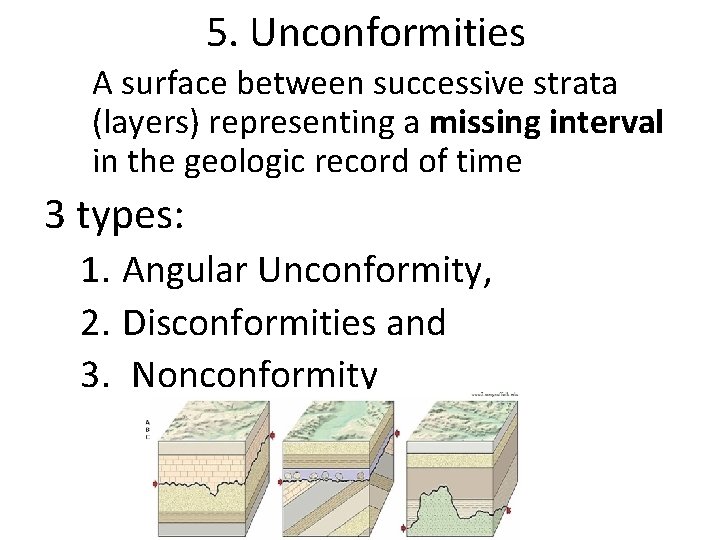
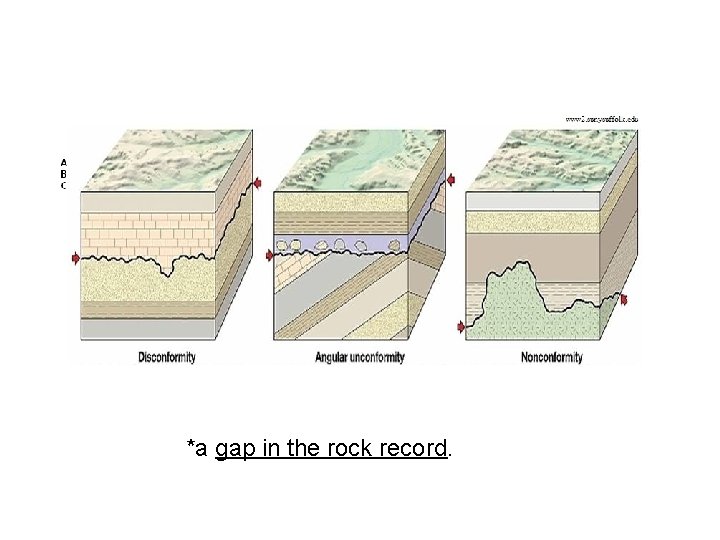
5. Unconformities A surface between successive strata (layers) representing a missing interval in the geologic record of time 3 types: 1. Angular Unconformity, 2. Disconformities and 3. Nonconformity

*a gap in the rock record.

6. Fossils - Correlation • Hand out fossils • Fossils are used to match up rock layers between widely separated areas or between continents • any time period can be recognized by its fossil content. • the matching up of rock layers from one area to another.

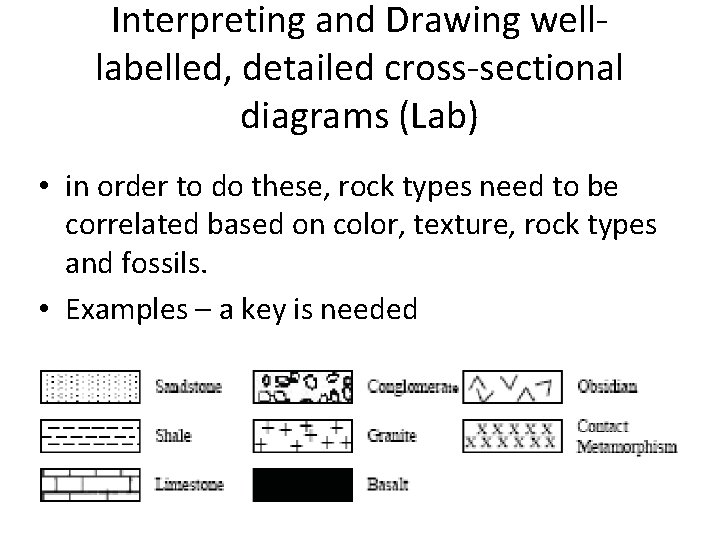
Interpreting and Drawing welllabelled, detailed cross-sectional diagrams (Lab) • in order to do these, rock types need to be correlated based on color, texture, rock types and fossils. • Examples – a key is needed

Absolute Dating - pg. 228 -235 • Is finding the exact age of a mineral, rock, fossil, landform or finding exactly when a geological event occurred. • 3 Ways ● 1. Tree Growth Rings 2. Varves - Glacial deposits 3. Radiometric dating ALL PRODUCES NUMBERS!

Tree Growth Rings • 1 Growth Ring = 1 Year • In places where seasonal changes occur (e. g. Newfoundland), plants add on a new growth ring each year. • By simply counting the growth rings in a tree, one can calculate its age.

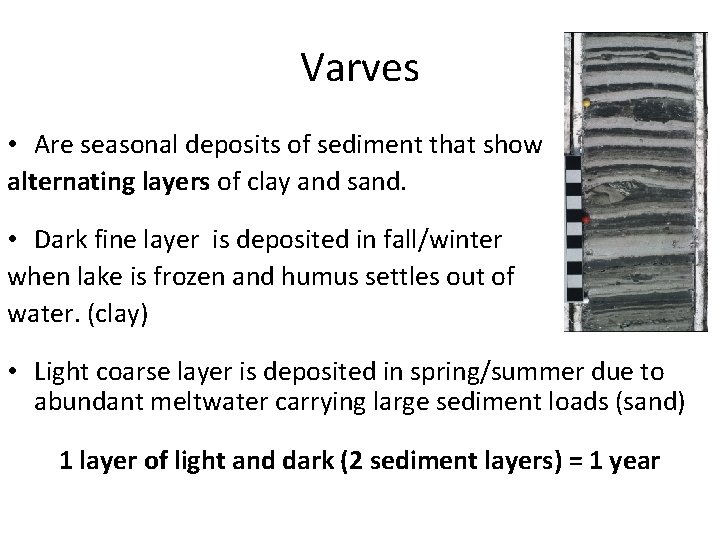
Varves • Are seasonal deposits of sediment that show alternating layers of clay and sand. • Dark fine layer is deposited in fall/winter when lake is frozen and humus settles out of water. (clay) • Light coarse layer is deposited in spring/summer due to abundant meltwater carrying large sediment loads (sand) 1 layer of light and dark (2 sediment layers) = 1 year

Radiometric Dating • Method of calculating the absolute age of minerals, rocks and fossils that contain radioactive isotopes

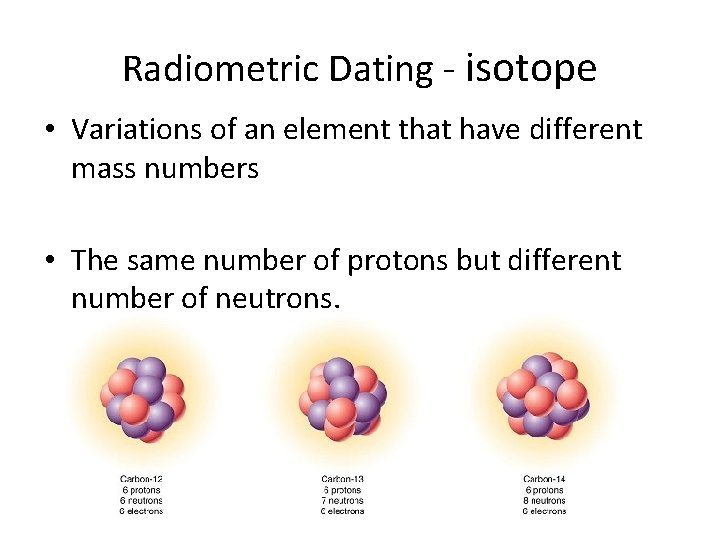
Radiometric Dating - isotope • Variations of an element that have different mass numbers • The same number of protons but different number of neutrons.

Radioactive Isotopes • Are unstable isotopes • The nucleus breaks down so the original isotope called the “Parent Material” decays into a new stable isotope called the “Daughter Product”. • The rate at which the nucleus breaks down is called “Half-Life”.

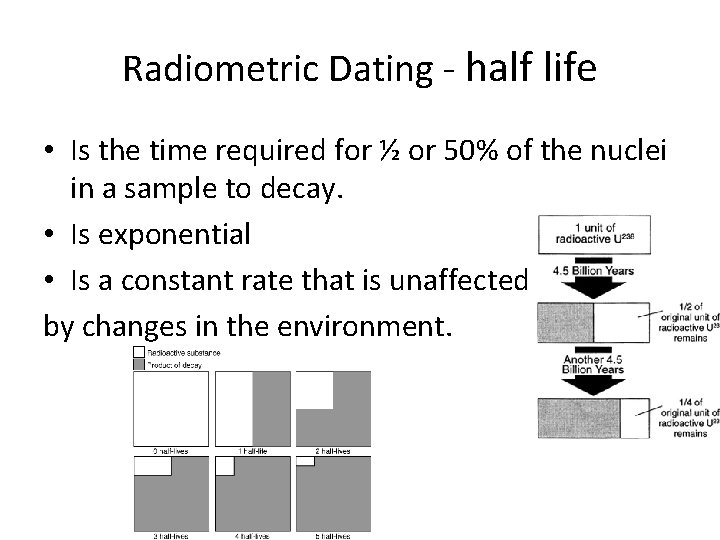
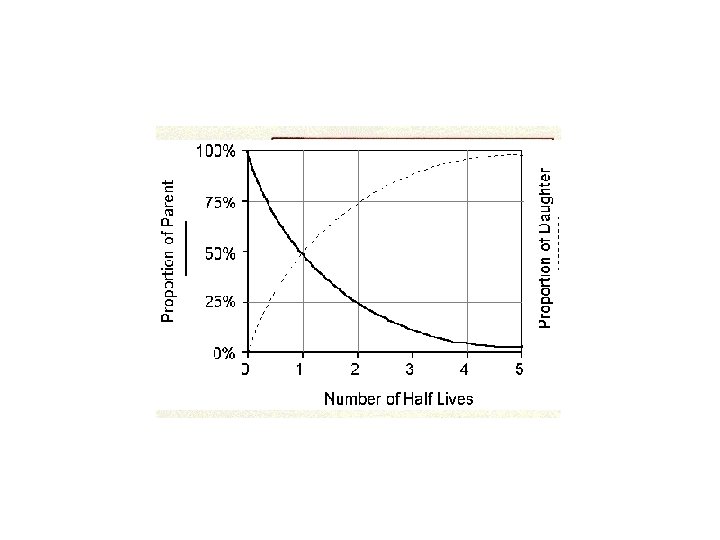
Radiometric Dating - half life • Is the time required for ½ or 50% of the nuclei in a sample to decay. • Is exponential • Is a constant rate that is unaffected by changes in the environment.

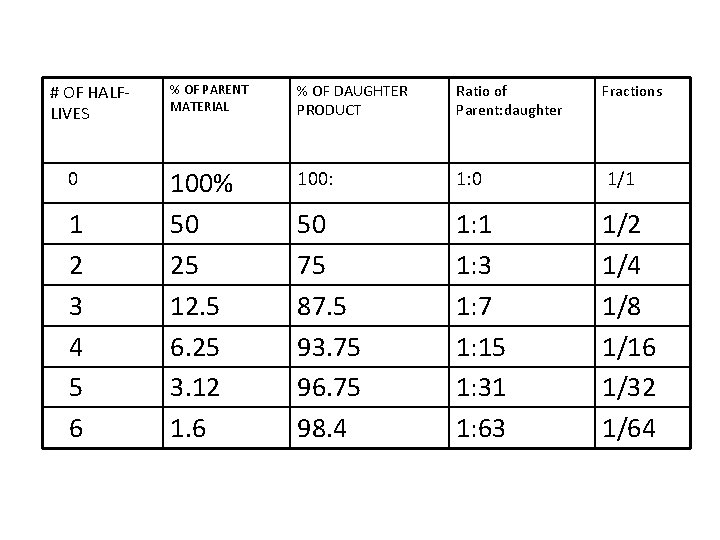
# OF HALFLIVES 0 1 2 3 4 5 6 % OF PARENT MATERIAL % OF DAUGHTER PRODUCT Ratio of Parent: daughter Fractions 100% 50 25 12. 5 6. 25 3. 12 1. 6 100: 1: 0 1/1 50 75 87. 5 93. 75 96. 75 98. 4 1: 1 1: 3 1: 7 1: 15 1: 31 1: 63 1/2 1/4 1/8 1/16 1/32 1/64


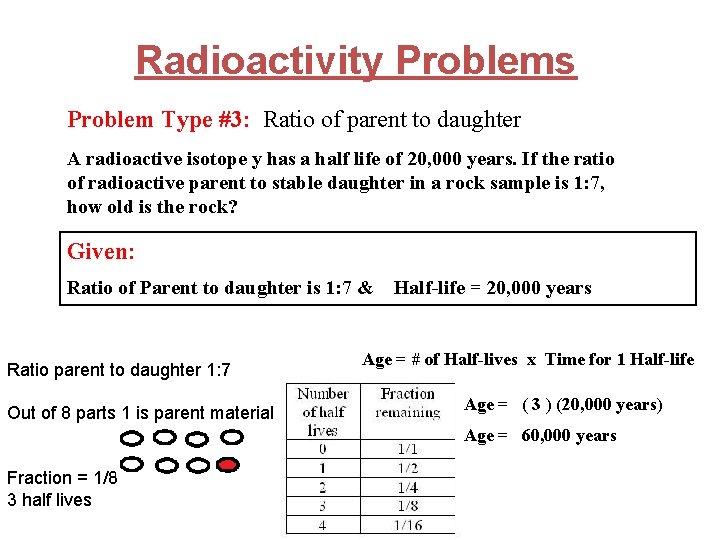
Radioactivity Problems Problem Type #3: Ratio of parent to daughter A radioactive isotope y has a half life of 20, 000 years. If the ratio of radioactive parent to stable daughter in a rock sample is 1: 7, how old is the rock? Given: Ratio of Parent to daughter is 1: 7 & Half-life = 20, 000 years

Radioactivity Problems Problem Type #3: Ratio of parent to daughter A radioactive isotope y has a half life of 20, 000 years. If the ratio of radioactive parent to stable daughter in a rock sample is 1: 7, how old is the rock? Given: Ratio of Parent to daughter is 1: 7 & Ratio parent to daughter 1: 7 Out of 8 parts 1 is parent material Half-life = 20, 000 years Age = # of Half-lives x Time for 1 Half-life Age = ( 3 ) (20, 000 years) Age = 60, 000 years Fraction = 1/8 3 half lives

Sources of Error pg. 231 1. Addition or loss of either the parent material or daughter product 2. Not for sedimentary rocks directly since it determines how long a go liquid rock cooled to a solid 3. Metamorphism resets the radioactive clock 4. Appropriate application of certain parentdaughter pairs. (to short/long half life)

Fossils What is a fossil? Fossils are the remains or traces of organisms found in sedimentary rocks. What conditions are necessary for fossils to form? 1. Rapid Burial 2. Presence of Hard Body Parts 3. Low Oxygen Environment

Methods of Fossilization Fossils are preserved in the rock record in several ways! 1) Petrification (or Petrifaction) By Replacement 2) Carbonization 3) Mold and Cast 4. Preservation (Intact) ● 5. Ice, Mummification, and Amber Traces (Indirect Evidence) ● Tracks, Burrows/Tunnels, Eggs, gastrolites (stomach stones), and Coprolites (feces).

Petrification Occurs when the small internal cavities and pores of the original structure are filled with precipitated mineral matter. ● ● Formation: ● ● cell walls and solid material are removed It is replaced by mineral material carried by ground water. The process is called replacement. Sometimes internal details and structures are retained.

Carbonization ● Formation: ●Fine sediment encloses delicate matter such as leaves (e. g. ferns) in a oxygen-poor environment. ●As time passes, pressure squeezes out the liquid and gaseous components of the organism leaving behind a thin residue of carbon.

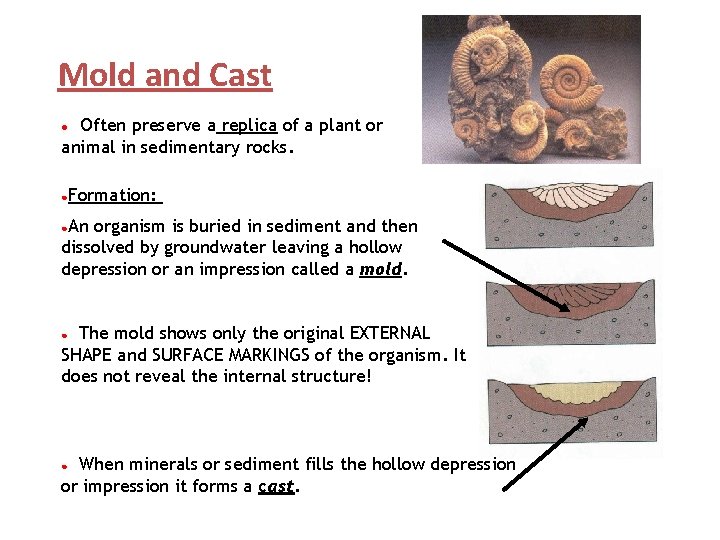
Mold and Cast Often preserve a replica of a plant or animal in sedimentary rocks. ● ●Formation: ●An organism is buried in sediment and then dissolved by groundwater leaving a hollow depression or an impression called a mold. The mold shows only the original EXTERNAL SHAPE and SURFACE MARKINGS of the organism. It does not reveal the internal structure! ● When minerals or sediment fills the hollow depression or impression it forms a cast. ●

Preservation Original remains can be preserved in ice or in amber (hardened tree sap). ● ● Formation: ●Either ice or amber covers the organism quickly after dying – or alive ●It is protected from decay (oxygen-free environment) and from pressures that would cause it to be crushed. The entire organism has been preserved; even the soft parts, which usually decay and disappear. ● Examples: (1) Woolly Mammoths preserved in ice in Alaska and Siberia. (2) Insects preserved in tree sap (amber). Cane in Jurassic Park.

Trace Fossils Show traces left in the rock by an organism. ● Formation: ●Not the organism itself ●Imprints or wastes left by the organism Examples include: Tracks - animal footprints made in soft sediment. The sediment later turns into sedimentary rock. Burrows/Tunnels - Animal trails made in soft sediment. The sediment later turns into sedimentary rock. Coprolites - Fossil dung (feces). Gastrolites – Stomach stones.

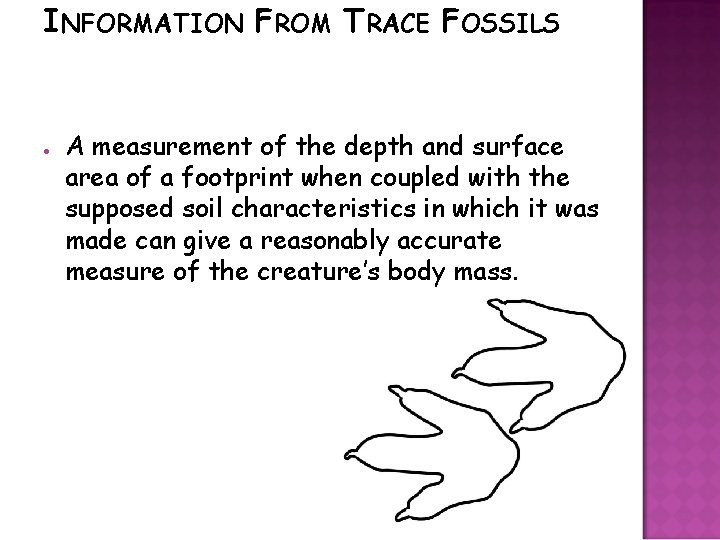
INFORMATION FROM TRACE FOSSILS ● A measurement of the depth and surface area of a footprint when coupled with the supposed soil characteristics in which it was made can give a reasonably accurate measure of the creature’s body mass.

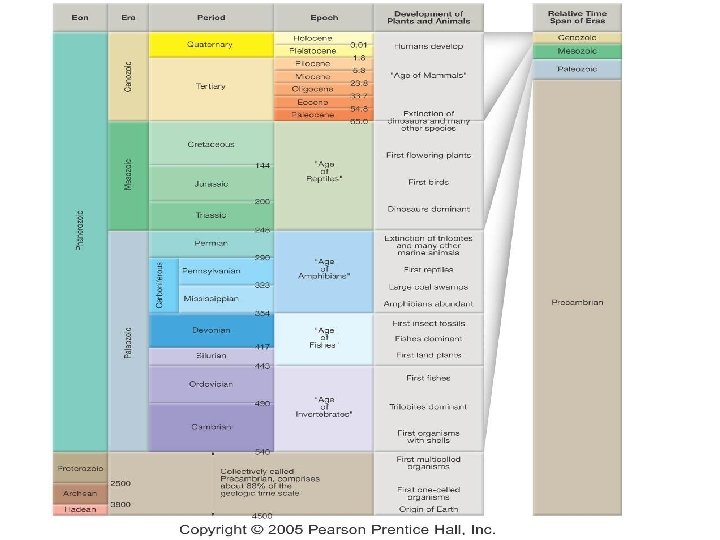
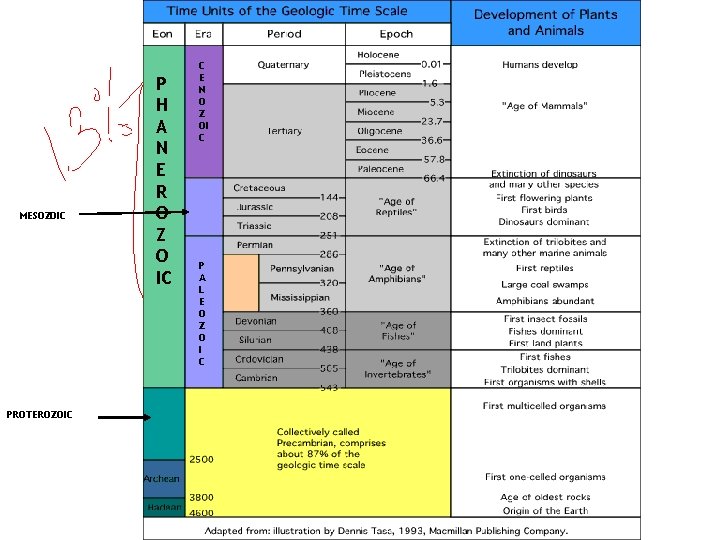
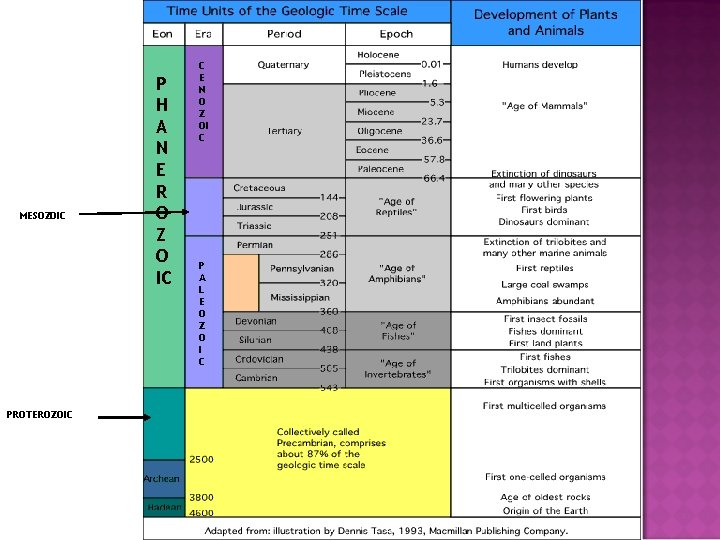
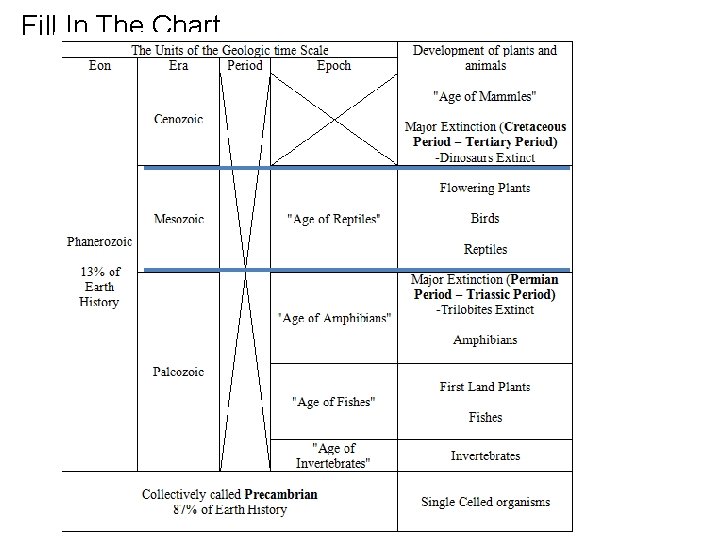
GEOLOGIC TIME ● ● ● Geologic time scale spans 4. 5 billion years. The last ~500 million years are detailed due to the study of fossils. Fossils have been used to divide geologic time into eons, eras, periods, and epochs.

Geologic Time Scale A Historical Perspective Scientist and their contributions to the Geologic Time Scale: Nicolaus Steno ● Principle of Original Horizontality. ● Principle of Superposition. James Hutton and Charles Lyell ● Principle of Uniformitarianism William Smith ● Principle of Faunal (Fossil) Succession


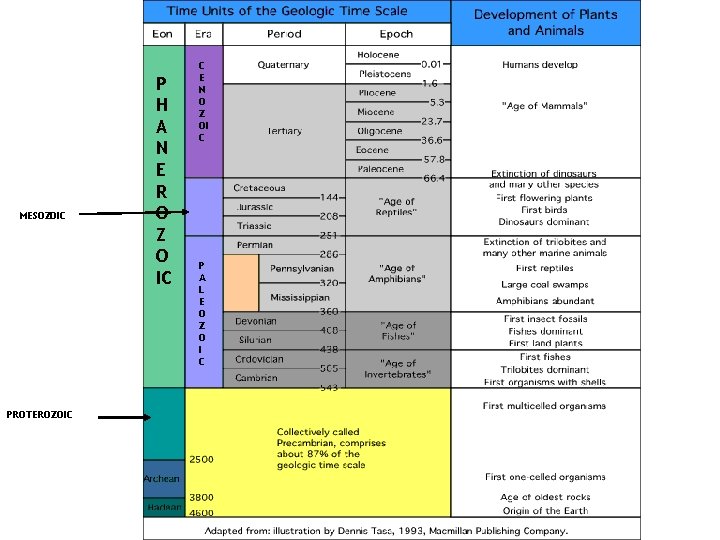
MESOZOIC PROTEROZOIC P H A N E R O Z O IC C E N O Z OI C P A L E O Z O I C

Geologic Time Scale What do the divisions of the geologic time scale signify? Eons Eras Divisions of Geologic Time Eon, Era, Period, Epoch Largest span of time Smallest span of time

EONS- largest time frames ● ● Don’t need to know the Eons in Precambrian (just collectively called Precambrian) Phanerozoic (“visible life”) — The most recent eon, which began about 540 million years ago. ● Fossils here help us determine the ages ● About 13% of Earth history. ● It represents the emergence of more complex life as organisms evolved.

MESOZOIC PROTEROZOIC P H A N E R O Z O IC C E N O Z OI C P A L E O Z O I C

Names of the Eras ● ● ● Era—Subdivision of an eon. Eras of the Phanerozoic eon include: ● Cenozoic (“recent life”) ● Mesozoic (“middle life”) ● Paleozoic (“ancient life”) Eras are subdivided into periods. Periods are subdivided into epochs. You do NOT need to know the periods or epochs. Do NOT confuse the Paleozoic Era with the Phanerozoic Eon.

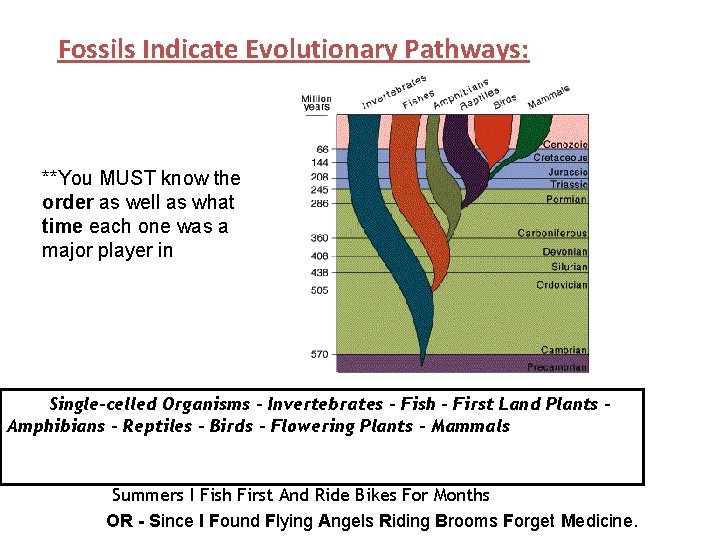
FOSSILS INDICATED EVOLUTIONARY PATHWAYS: Each Era can be divided based on the fossil evidence found in rocks during those times Precambrian Era – “single celled organisms” Little direct evidence of fossils due to lack of species with hard body parts (and thus lack of lifeforms). ● Fossil evidence include algae, bacteria, and traces of soft-bodied organisms. ●

MESOZOIC PROTEROZOIC P H A N E R O Z O IC C E N O Z OI C P A L E O Z O I C

● FOSSILS INDICATE EVOLUTIONARY PATHWAYS: Early Paleozoic Era -- “Age of the Invertebrate” Paleozoic Divided Invertebrates evolved into. Era vertebrates. ● ● ● Middle Paleozoic Era – “Age of Fishes” ● Abundance of fishes and First land plants Late Paleozoic -- “age of amphibians”. ● Lung fish evolved into amphibians ● Mass extinctions of invertebrates including trilobites and numerous other marine species occurred at the end of the Paleozoic Era.

Fossils Indicate Evolutionary Pathways: ● Mesozoic Era -- “Age of the Reptiles” ● Reptiles - Dinosaurs became dominant. ● First birds are seen during this time. ● First Flowering Plants The end of the Mesozoic Era was marked by mass extinctions of reptiles including dinosaurs and numerous other species. Meteorite! Cenozoic Era -- “Age of the Mammals” ● ● ● Mammals evolve and dominate during this time.

Fossils Indicate Evolutionary Pathways: **You MUST know the order as well as what time each one was a major player in Single-celled Organisms - Invertebrates – Fish – First Land Plants – Amphibians – Reptiles – Birds – Flowering Plants - Mammals Summers I Fish First And Ride Bikes For Months OR - Since I Found Flying Angels Riding Brooms Forget Medicine.

Mnemonic device ● ● ● ● ● Summers I Fish First And Ride Bikes For Months S = Single-celled organisms (Precambrian) I = Invertebrates (Early Paleozoic -1/4) F = Fishes (Middle Paleozoic -2/4) F = First Land Plants (Early To Middle Paleozoic -3/4) A = Amphibians (Late Paleozoic – 4/4) R = Reptiles (Mesozoic – 1/3) B = Birds (Mesozoic – 2/3) F = Flowering Plants (Mesozoic -3/3) M = Mammals (Cenozoic)

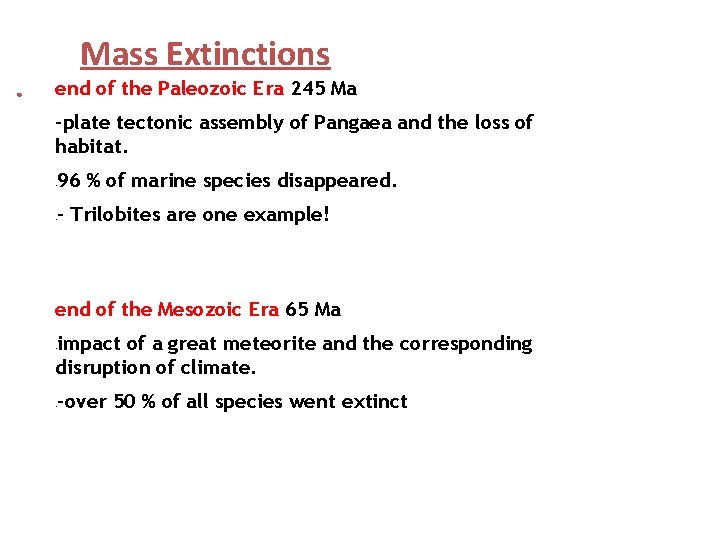
Mass Extinctions 1) Permian Period – Triassic Period Boundary (End of Paleozoic Era and Beginning of Mesozoic Era) 2) Cretaceous Period – Tertiary Period Boundary (End of Mesozoic Era and Beginning of Cenozoic Era) Some species flourished as other species went extinct!

Mass Extinctions ● end of the Paleozoic Era 245 Ma -plate tectonic assembly of Pangaea and the loss of habitat. 96 % of marine species disappeared. - - Trilobites are one example! - end of the Mesozoic Era 65 Ma impact of a great meteorite and the corresponding disruption of climate. - -over 50 % of all species went extinct -

Fill In The Chart






There are over 100 known elements, but only 8 of them make up over 98. 5% of the earths crust by mass. 1) Oxygen (46. 6%) 3) Aluminum (8. 1%) 5) Calcium (3. 6%) 7) Potassium (2. 6%) 2) Silicon (27. 7%) 4) Iron (5. 0%) 6) Sodium (2. 8%) 8) Magnesium (2. 1%)

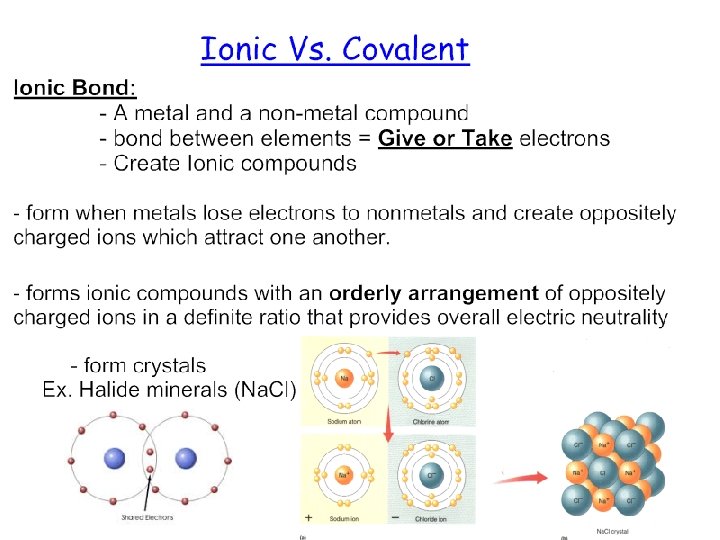
Definition of “Mineral” pg. 32 Minerals are the building blocks of rocks. • Must exhibit specific characteristics: 1. Must occur naturally. 2. Must be inorganic ( not from living organisms) 3. Must be solid. 4. Must have a crystal structure 5. Must have a definite chemical composition ( chemical formula)

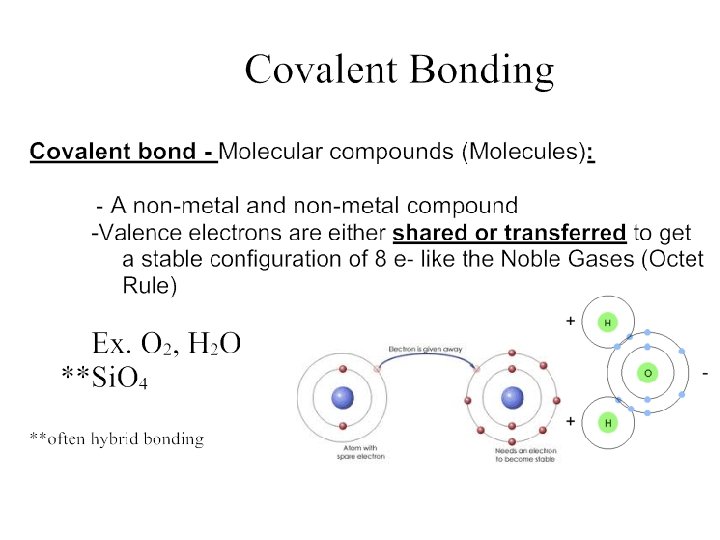
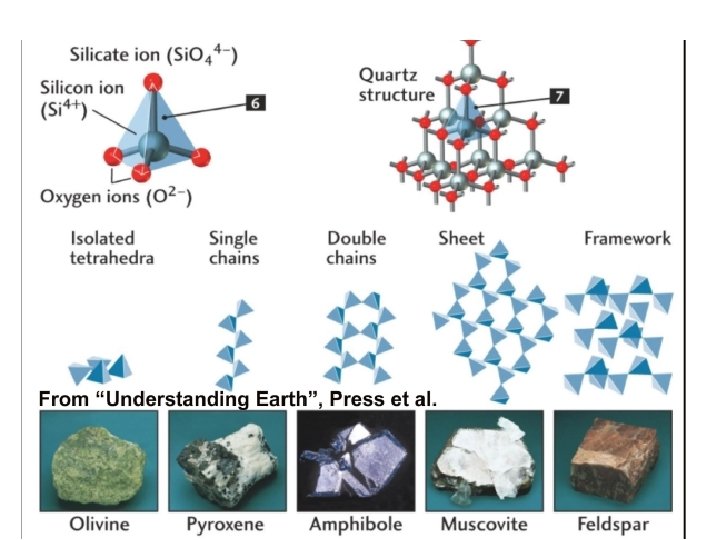
Mineral Groups Minerals that form the rocks within Earth’s crust belong to seven (7) main mineral groups, which include: 1) Silicates 2) Carbonates 3) Sulfates 4) Oxides 5) Halides 6) Sulfides 7) Native Elements

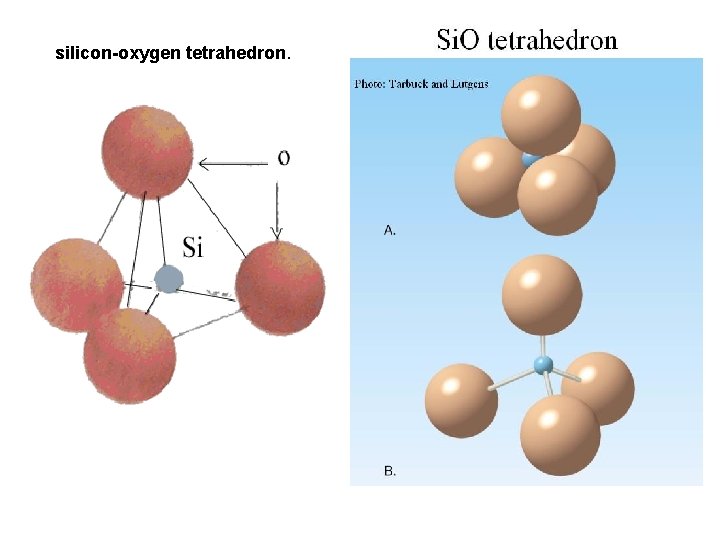
silicon-oxygen tetrahedron.

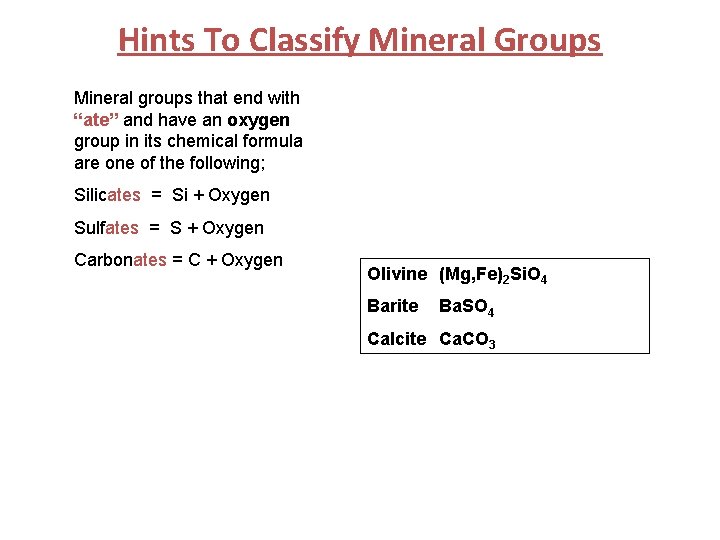
Hints To Classify Mineral Groups Mineral groups that end with “ate” and have an oxygen group in its chemical formula are one of the following; Silicates = Si + Oxygen Sulfates = S + Oxygen Carbonates = C + Oxygen Olivine (Mg, Fe)2 Si. O 4 Barite Ba. SO 4 Calcite Ca. CO 3

Hints to Classify Mineral Groups Mineral groups that end with “ide” and have a metal (e. g. , Na, K) in its chemical formula are one of the following; Oxides = Metal + O Sulfides = Metal + S Halides = Metal + Cl, Br, F Hematite Fe 2 O 3 Pyrite Fe. S 2 Fluorite Ca. F 2

Mineral Groups 1 Silicates v A mineral group that has silicon and oxygen as part. atomic structure. (Si. Ox) Rock forming silicates are divided into two groups: Sialic Silicates (Aluminosilicates) Ø Rich in silicon and aluminum. Ø Mineral are light in color. Simatic Silicates Ø Rich in silicon and magnesium. Ø Minerals are dark in colour. Examples include: Quartz Mica (Muscovite) Feldspar Examples include: Olivine Pyroxene of their

Mineral Groups 2 Carbonates v compounds consisting of an atomic structure of one carbon and three oxygen (CO 3). vcalcite (Ca CO 3), 3 Sulfates v compounds consisting of an atomic structure of one sulfur and four oxygen (SO 4). v the rock gypsum v. The mineral barite (Ba. SO 4) is mined and used in drilling mud.

Mineral Groups 4 Oxides v compounds consisting of an atomic structure of oxygen combined with one or more metals. v. Ore Minerals. As an example, the mineral hematite is Fe 2 O 3 5 Halides v compounds consisting of an atomic structure of chlorine (Cl), bromine (Br) or fluorine (F) with sodium, potassium, or calcium. v Halite (Na. Cl) is the most common halide. It is often referred to as table salt.

Mineral Groups 6 Sulfides v compounds consisting of an atomic structure of one or more metals combined with sulfur. vpyrite (Fe. S 2), galena (Pb. S), and sphalerite (Zn. S). 7 Native Minerals v elements that occur uncombined in nature. v commonly called native elements. v examples include: gold (Au), silver (Ag), copper (Cu), and sulfur (S).

NOTE • Note that an ore mineral is any mineral that has enough of a particular element in it to be mined at a profit.

Why should we care? Nearly all manufactured products we use are obtained from minerals. èaluminum: soft drink cans ègraphite (carbon): our pencil lead ècopper: wire for our electricity ètalc: baby powder èsilver & gold: our jewelry èsilicon: our computer chips Cheat sheet

Speaking of Atomic Arrangement (Structure) • Consider diamond versus graphite! • Note that completely different minerals can form from the same atom, depending on how the atoms are arranged. • ↑ pressure = closer packing of atoms = different substance. • Temperature and pressure conditions under which minerals form are very important.

Diamond Versus Graphite • Diamond and graphite are polymorphs of the element carbon; however, they differ in terms of the mineral properties hardness and cleavage due to arrangement of the carbon atoms. • Diamond is hard and has no cleavage since the carbon atoms are arranged in a network covalent structure. This does not allow for any weak planes of bonding. • Graphite is soft and has perfect basal cleavage (sheets) since the carbon atoms are arranged in planes of strong bonding with planes of weak bonding in between.

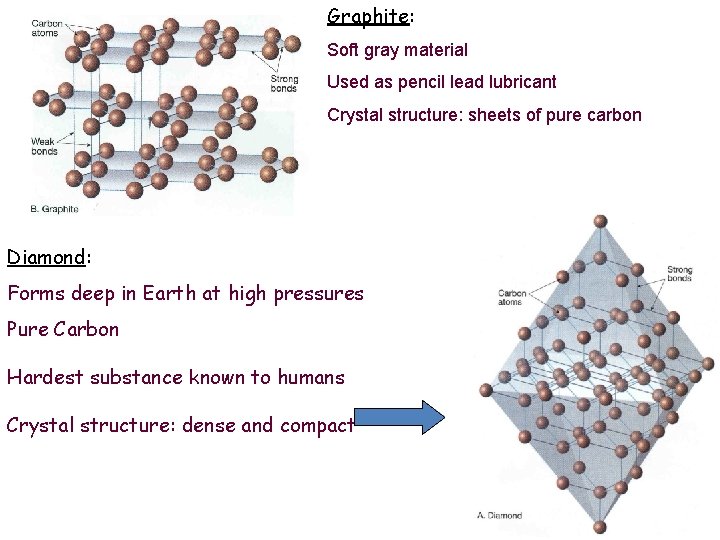
Graphite: Soft gray material Used as pencil lead lubricant Crystal structure: sheets of pure carbon Diamond: Forms deep in Earth at high pressures Pure Carbon Hardest substance known to humans Crystal structure: dense and compact

Mineral Properties Ø The following are a list of physical properties that minerals could display: 1) Specific Gravity – LATER so important its own class 2) Hardness 3) Cleavage Versus Fracture 4) Streak 5) Luster 6) Colour 7) Others Taste, Feel, Magnetism, Acid Test, Crystal Form, Smell, Double Refraction, Tenacity, and Fluorescence.

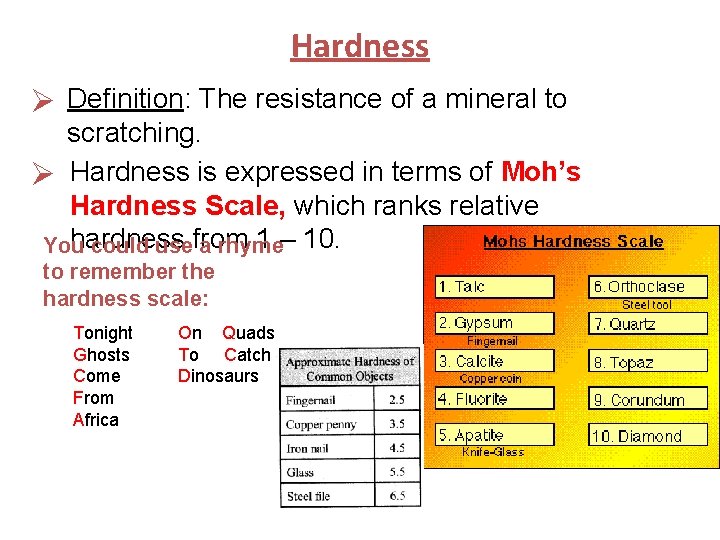
Hardness Ø Definition: The resistance of a mineral to scratching. Ø Hardness is expressed in terms of Moh’s Hardness Scale, which ranks relative hardness 1 – 10. You could usefrom a rhyme to remember the hardness scale: Tonight Ghosts Come From Africa On Quads To Catch Dinosaurs

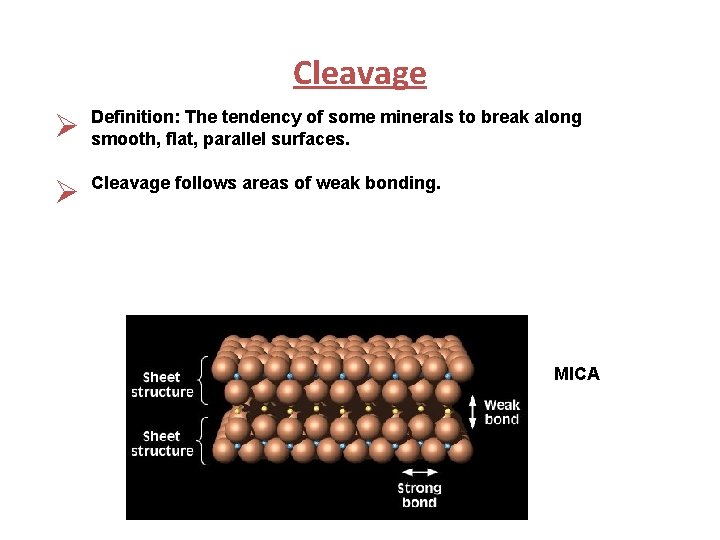
Cleavage Ø Definition: The tendency of some minerals to break along smooth, flat, parallel surfaces. Ø Cleavage follows areas of weak bonding. MICA

Cleavage Plane Directions Cleavage in one direction is called basal cleavage. Example: Mica Cleavage in two directions. Example: Orthoclase feldspar displays this type of cleavage Cleavage in three directions. Examples: Halite, Galena, and Pyrite.

Ø Fracture A mineral that do not have any cleavage planes is said to break by “Fracture“, which is the tendency of a mineral to break irregularly. Example is glass or the mineral quartz, which is said to have Conchoidal Fracture. This is a curved breakage that resembles the concentric shape of a mussel shell.

Streak Ø The true color of the mineral. It is the colour of the mineral in its powdered form. Ø To find the streak of a mineral, you must perform a streak test. To do this, you scratch a mineral across an unglazed porcelain tile and the powder streak left on the tile is the true color of the mineral.

Luster Ø Ø The appearance of the mineral in reflected light. Most minerals can be described as either: 1. Metallic OR 2. Non-Metallic A) Glassy B) Greasy C) Earthy or Dull D) Pearly

Colour Ø Ø The actual colour of the mineral that you see. Ø Three reasons: This property is less distinctive. reliable as the others) (not as WHY? 1) Different minerals can have the same color. 2) Some minerals may have impurities, which cause a single mineral to have different colors. 3) Surface Oxidation

Other Properties These properties can be helpful to identify minerals that are similar:

Other Properties • Double Refraction: This is an optical property. For example, when a transparent piece of calcite is placed over printed material, the letters appear double.

Other Properties • Tenacity: • Mica (muscovite and biotite) will bend and elastically snap back. • Gold is malleable, which means that it can be hammered into sheets.

Other Properties • Crystal Form (Shape): Shape or form of a crystal can reflect the orderly internal arrangement of atoms.

Crystal Faces The smooth flat surfaces on crystals are called faces.

Other Properties • Fluorescence: When light from a source strikes a mineral and reacts with the component chemicals, thereby making the mineral glow. Example: Gypsum

Specific Gravity Ø Specific Gravity (SG): is the mass of a mineral compared to that of an equal volume of water. To determine specific gravity, you need to carry out the following three steps: 1) Weigh the specimen in air and record the weight.

Specific Gravity 2) Weigh the specimen submerged in water and record the weight.

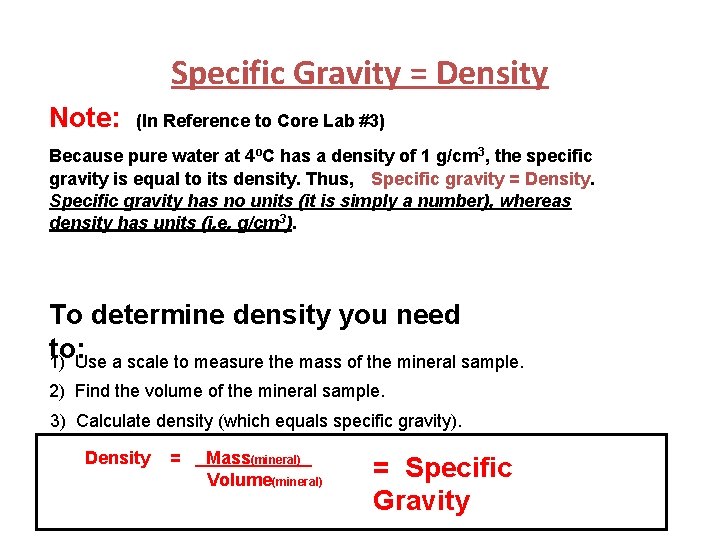
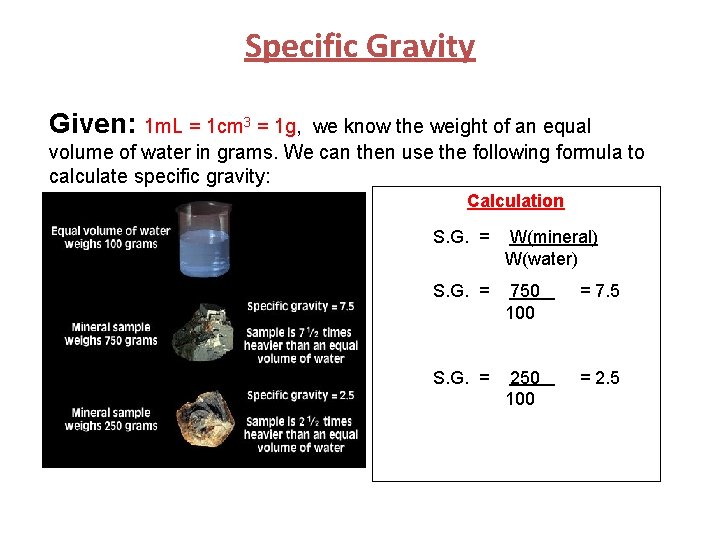
Specific Gravity = Density Note: (In Reference to Core Lab #3) Because pure water at 4ºC has a density of 1 g/cm 3, the specific gravity is equal to its density. Thus, Specific gravity = Density. Specific gravity has no units (it is simply a number), whereas density has units (i. e. g/cm 3). To determine density you need to: 1) Use a scale to measure the mass of the mineral sample. 2) Find the volume of the mineral sample. 3) Calculate density (which equals specific gravity). Density = Mass(mineral) Volume(mineral) = Specific Gravity

Specific Gravity Given: 1 m. L = 1 cm 3 = 1 g, we know the weight of an equal volume of water in grams. We can then use the following formula to calculate specific gravity: Calculation S. G. = W(mineral) W(water)

Specific Gravity Given: 1 m. L = 1 cm 3 = 1 g, we know the weight of an equal volume of water in grams. We can then use the following formula to calculate specific gravity: Calculation S. G. = W(mineral) W(water) S. G. = 750 100 = 7. 5 S. G. = 250 100 = 2. 5

Rocks and The Rock Cycle • A rock is a solid mixture of one or more minerals. • The picture below shows a piece of granite, which is a go-to example of a rock

Difference between a mineral and a rock? • Minerals tend to have a characteristic crystal structure based on chemical composition, • Rocks have many minerals in them, so they do not have a definite structure. – Granite (rock) is made of feldspar, quartz, amphibole, mica, pyroxene, etc (all minerals).

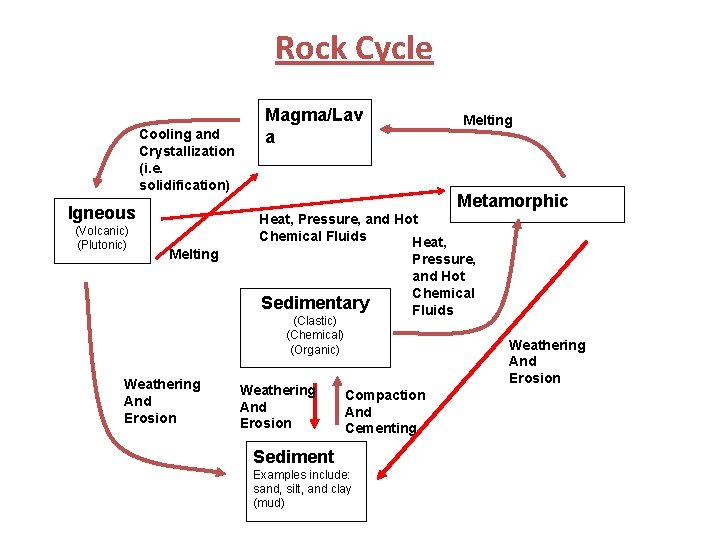
Rocks and the Rock Cycle Ø The Three Rock Types Include: 1) Igneous Rocks (i. e. Fire Rocks – from molten material) 2) Sedimentary Rocks (i. e. Layered Rocks – from deposition) 3) Metamorphic Rocks (i. e. Changed Rocks) Ø All three rock types are interrelated through the rock cycle. The three rock types are classified by their nature of origin (i. e. formation). Reference: Tarbuck and Lutgens Pages 15 - 17

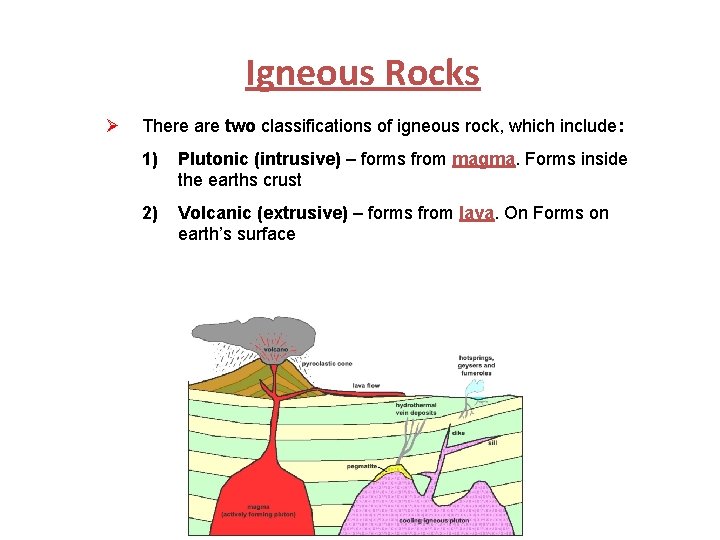
Igneous Rocks Ø Rocks that have solidified from a molten state. Ø If the molten material is located below Earth’s surface, then it is called magma. Magma is higher in gases than lava. Ø If the molten material is located on Earth’s surface, then it is called lava. Lava is lower in gases than magma.

Igneous Rocks Ø There are two classifications of igneous rock, which include: 1) Plutonic (intrusive) – forms from magma. Forms inside the earths crust 2) Volcanic (extrusive) – forms from lava. On Forms on earth’s surface

Extrusive Vs. Intrusive Igneous Rocks • Extrusive are on the surface - cool quickly, no time to form, so have small grains • Intrusive are in the earth - cool slowly, have time to form so have larger grains

Sedimentary Rocks Ø Rocks formed when the weathered products (i. e. sediment) of pre-existing rocks have been transported, deposited, compacted, and cemented into solid rock. Ø These rocks usually show layering/strata/beds. Ø Most of Earth’s crust (95%) is igneous rocks; however, the surface of the crust is largely covered by sedimentary rocks.

• Clastic (i. e. Detrital) sedimentary Rocks: Formed by deposition of rock and mineral fragments Chemical sedimentary Rocks: formed from minerals that were carried in solution (dissolved in water) and are deposited as precipitates. Organic (Biogenic) Sedimentary Rocks: formed from the remains of dead animals.

Metamorphic Rocks Ø Rocks formed below Earth’s surface when pre-existing rocks (i. e. igneous, sedimentary, and even metamorphic) are altered Ø 3 Agents of Metamorphisism: 1. heat, 2. pressure, and 3. chemically active fluids (e. g. water). NOTE: MELTING IS NOT INVOLVED IN THE PROCESS OF METAMORPHISM.

Metamorphic Rocks • Examples of metamorphic rocks include: Quartzite, Marble, Slate, Phyllite, Schist, and Gneiss. • Note that there are two types of metamorphism, which include: – Contact and Regional.

Pre-notes on the Rock Cycle • A) All three types of rocks could be weathered and eroded compacted into sedimentary layers. • B) All three types of rocks could be metamorphosed if subjected to appropriate conditions of metamorphism (for example: heat, pressure, chemically-active fluids). • C) All three types of rocks could be melted if the temperatures become high enough. • D) Once melting to a molten has occurred, igneous activity has begun. • E) The process of melting is not involved in metamorphism. If it melts, it becomes liquid rock, which will then cool to form Igneous Rock.

Rock Cycle Cooling and Crystallization (i. e. solidification) Igneous (Volcanic) (Plutonic) Melting Magma/Lav a Metamorphic Heat, Pressure, and Hot Chemical Fluids Heat, Sedimentary (Clastic) (Chemical) (Organic) Weathering And Erosion Melting Weathering And Erosion Pressure, and Hot Chemical Fluids Weathering And Erosion Compaction And Cementing Sediment Examples include: sand, silt, and clay (mud)
 Earth systems 3209
Earth systems 3209 Scarcity entrepreneurship definition
Scarcity entrepreneurship definition Entrepreneurship 3209
Entrepreneurship 3209 Database systems midterm exam
Database systems midterm exam Operating systems midterm
Operating systems midterm Computer science midterm
Computer science midterm Introduction to computer science midterm exam
Introduction to computer science midterm exam Introduction to computer science midterm exam test
Introduction to computer science midterm exam test Computer science practice midterm
Computer science practice midterm My favorite subject is science แปลว่า
My favorite subject is science แปลว่า Amendments mun
Amendments mun Mun position paper
Mun position paper Honorable chair fellow delegates
Honorable chair fellow delegates Mun dress code
Mun dress code Model un motions
Model un motions Mun engineering complementary studies
Mun engineering complementary studies Mun sign in
Mun sign in Tuen mun golf
Tuen mun golf Mun kyong-jin
Mun kyong-jin Operative clauses
Operative clauses Mun operative clauses
Mun operative clauses Model united nations
Model united nations Introduction to mun
Introduction to mun Chan ho mun
Chan ho mun Tuen mun hospital ambulatory care centre
Tuen mun hospital ambulatory care centre How mun works
How mun works Point of entertainment mun
Point of entertainment mun Mathematics iceberg
Mathematics iceberg Shad mun
Shad mun Seng mun kong
Seng mun kong Mun sgs
Mun sgs How to write a resolution mun
How to write a resolution mun Mun choon chan
Mun choon chan Mun 101
Mun 101 Chan park microsoft
Chan park microsoft Anatolia college mun
Anatolia college mun Model un position paper outlines
Model un position paper outlines Mun ugm
Mun ugm Flcs mun
Flcs mun Nlp midterm exam
Nlp midterm exam Data mining exam
Data mining exam Html midterm exam
Html midterm exam English 1 midterm exam
English 1 midterm exam Wearing a poppy bruise
Wearing a poppy bruise Business law midterm answers
Business law midterm answers Hippo hat mnemonic
Hippo hat mnemonic Algebra 1 midterm exam
Algebra 1 midterm exam Whap midterm review
Whap midterm review Eku dual credit
Eku dual credit Midterm exam for applied research methods
Midterm exam for applied research methods Prelim, midterm finals grading system
Prelim, midterm finals grading system Nr 601 midterm test bank
Nr 601 midterm test bank Ics 33 midterm
Ics 33 midterm Marketing midterm review
Marketing midterm review Mid term thesis presentation
Mid term thesis presentation Midterm break poem
Midterm break poem Global history midterm
Global history midterm Global 9 midterm review
Global 9 midterm review Biology practice midterm
Biology practice midterm English 9 midterm exam
English 9 midterm exam Trig midterm review
Trig midterm review Regents chemistry midterm
Regents chemistry midterm Chemistry midterm review
Chemistry midterm review Oligotrophic definition apes
Oligotrophic definition apes Ap english language and composition midterm exam
Ap english language and composition midterm exam Ap chemistry midterm exam
Ap chemistry midterm exam What is a midterm
What is a midterm Algebra 2 midterm study guide
Algebra 2 midterm study guide Grva midterm exam
Grva midterm exam Spanish 2 midterm practice test
Spanish 2 midterm practice test To remember a list of the school supplies she needs
To remember a list of the school supplies she needs Midterm makeup
Midterm makeup Midterm exam format
Midterm exam format American literature midterm exam
American literature midterm exam World history semester 1 exam
World history semester 1 exam English 11 midterm exam
English 11 midterm exam Cs 102 midterm
Cs 102 midterm Midterm report
Midterm report Geometry midterm exam
Geometry midterm exam Ratee definition
Ratee definition Exam midterm
Exam midterm Biology midterm review
Biology midterm review Airman comprehensive assessment
Airman comprehensive assessment Midterm exam announcement
Midterm exam announcement Physics practice midterm
Physics practice midterm Personal finance midterm
Personal finance midterm Midterm
Midterm Midterm paper
Midterm paper Seamus heaney midterm break
Seamus heaney midterm break College sick bay
College sick bay Midterm
Midterm Ipc144 midterm
Ipc144 midterm Pols 1101 final exam study guide
Pols 1101 final exam study guide Midterm for one for short
Midterm for one for short Comp 248 midterm
Comp 248 midterm Comp 248 midterm
Comp 248 midterm Web development midterm exam
Web development midterm exam Xiao team comp
Xiao team comp Government midterm
Government midterm Earth observing systems data analytics
Earth observing systems data analytics Big idea 7 study guide earth systems and patterns
Big idea 7 study guide earth systems and patterns Earth systems
Earth systems Big idea 7 earth systems and patterns
Big idea 7 earth systems and patterns Environmental science chapter 3
Environmental science chapter 3 Geosphere
Geosphere Earth's spheres foldable
Earth's spheres foldable Earth observing systems data analytics
Earth observing systems data analytics Hydrosphere
Hydrosphere The shape of the earth
The shape of the earth Decision support systems and intelligent systems
Decision support systems and intelligent systems Engineering elegant systems: theory of systems engineering
Engineering elegant systems: theory of systems engineering Embedded systems vs cyber physical systems
Embedded systems vs cyber physical systems Elegant systems
Elegant systems Meteorological symbols for four types of fronts
Meteorological symbols for four types of fronts Earth science sol 2010
Earth science sol 2010 Earth science regents lab practical review
Earth science regents lab practical review Earth science reference table page 8 and 9
Earth science reference table page 8 and 9 Earth science regents lab practical
Earth science regents lab practical Earth science grade 9
Earth science grade 9 Dynamic equilibrium earth science
Dynamic equilibrium earth science Hydrosphere includes
Hydrosphere includes Earth science jeopardy 8th grade
Earth science jeopardy 8th grade Geology earth science definition
Geology earth science definition Final exam environmental science
Final exam environmental science Earth science meaning
Earth science meaning Astronomy definition earth science
Astronomy definition earth science Earth science sol
Earth science sol Earth science vs geology
Earth science vs geology Earth science final exam review
Earth science final exam review Earth science lab practical
Earth science lab practical Earth science semester 2 final exam answers
Earth science semester 2 final exam answers Streak definition earth science
Streak definition earth science Vertical structure of the earth
Vertical structure of the earth Relative humidity reference table
Relative humidity reference table 4 major branches of science
4 major branches of science 282 ways to pass the earth science regents
282 ways to pass the earth science regents