Earth Science Notes Classification of Matter Classification of









- Slides: 12

Earth Science Notes Classification of Matter

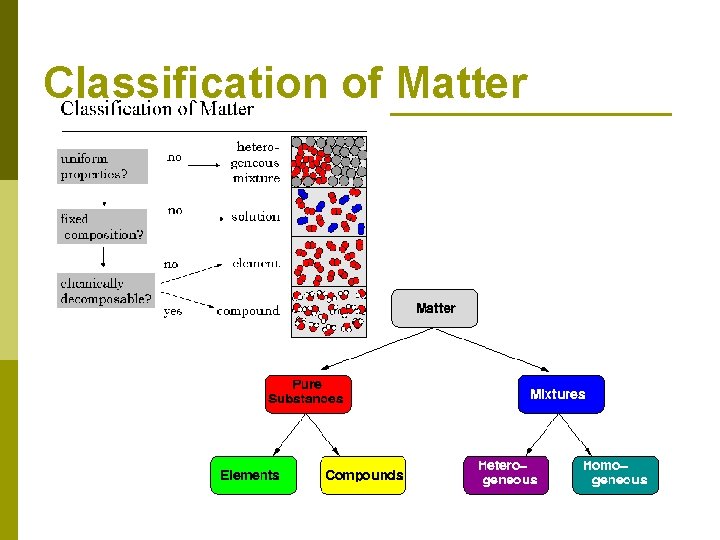
Classification of Matter is classified by its composition (what makes it up) p Matter that has a definite composition is called a pure substance p Two types of pure substances: p n n Elements: made up of only one kind of atom Compounds: made up of two or more elements (kinds of atoms)

Classification of Matter that has a variable composition is called a mixture. p Two types of mixtures: p n Homogeneous mixture: evenly mixed solution. p Solutions: a homogeneous mixture § Solute: the substance being dissolved § Ex: salt in water § Solvent: the substance that does the dissolving § Ex: water

Classification of Matter Two types of mixtures: (cont. ) p Heterogeneous mixture: unevenly mixed solution p n Colloids: a heterogeneous mixture that never settles Ex: Milk p Tyndall Effect: the scattering of light by a mixture p n Suspension: a heterogeneous mixture where the particles are visible and settle.

Classification of Matter

Describing Matter Properties: characteristic about a substance or a mixture. p Question: Why do scientists care about properties of matter? p Answer: Properties help scientist to determine the identity of certain materials as well as helping them determine what type of changes have occurred in the material. p

Describing Matter Types of Properties: p Physical Property: a property that can be observed that does not alter the make-up of the material. n Example of physical properties p p Mass, volume, density Shape Color State (solid, liquid, gas) § Boiling, Melting points p Magnetic p Odor

Describing Matter Types of Properties: (cont. ) p Chemical Property: a property of a substance that determines how it will interact with other agents n Example of chemical properties Flammability p Reactivity p Bonding tendencies p § How a substance reacts in the presence of other substances p Radioactivity

Changes in Matter Physical change: a change in the size, state or shape of matter. The identity of a material stays the same. Examples p Ex: ice thaws water; water evaporates water vapor. n p No matter the state always H 2 O Ex: Paper being cut into small pieces n Start with paper after physical change still have paper.

Changes in Matter Chemical change: the changing of one material into another. Examples p EX: water is decomposed into hydrogen and oxygen via an electrical current. n p Start with H 2 O end with H 2 and O 2 Ex: burn paper and it is not paper any more. n You get carbon, water, and carbon monoxide.

Changes in Matter The Law of Conservation of Matter: matter cannot be created nor destroyed but can be changed from one form to another. p Application to changes in matter: the amount of mass you have before a reaction has to equal the amount of mass after the reaction. p

Summary Be able to… p Classify matter substance, mixture, etc. p Describe matter using properties p Describe, Classify, and explain changes matter undergoes.