Earth Science 2 2 Minerals Minerals A mineral























- Slides: 24

Earth Science 2. 2 Minerals

Minerals � A mineral is a � naturally occurring, � inorganic solid � with an orderly crystalline structure � and a definite chemical composition.

Minerals � Materials must possess these 5 major characteristics to be considered a mineral 1. 2. 3. 4. 5. Naturally occurring Solid substance Orderly crystalline structure Definite chemical composition Inorganic in makeup

Characteristics of Minerals 1. Naturally occurring: � a mineral forms by natural geological processes. � Therefore, synthetic gems, such as synthetic diamonds and rubies, are not considered minerals

Characteristics of Minerals 2: Solid substance: � minerals are solids within the temperature ranges that are normal for Earth’s surface

Characteristics of Minerals 3: Orderly crystalline structure: � minerals are crystalline substances which means � “their atoms are arranged in an orderly and repetitive manner”.

Characteristics of Minerals 4: definite chemical composition: � most minerals are chemical compounds made of two or more elements. � A few, such as Gold or Silver, consist of only a single element when in native form.

Characteristics of Minerals 5: generally considered inorganic: � minerals are inorganic crystalline solids found in nature. (Inorganic means not coming from some type of living organism) � We say generally inorganic because coral reefs are formed from inorganic compounds secreted by marine animals. We consider this form of calcium carbonate a mineral even though it is a byproduct of a living organism.

How Minerals Form � Minerals form in � many locations on Earth � from a variety of different conditions.

How Minerals Form � Minerals called silicates often form deep in the Earth’s crust or mantle where temperatures and pressures are high. � Most minerals known as carbonates form in warm, shallow ocean waters.

How Minerals Form � Most clay minerals form at or near Earth’s surface when existing minerals are exposed to weathering. � Still other minerals form when rocks are subjected to changes in pressure or temperature.

How Minerals Form � 1. 2. 3. 4. There are four major processes by which minerals form: Crystallization from magma Precipitation Changes in pressure and temperature Formation from hydrothermal solutions

Crystallization from Magma � Magma is molten rock that forms deep within the Earth. � As magma cools, elements combine to form minerals. � The first minerals to crystallize from magma are usually those rich in iron, calcium and magnesium. � As minerals continue to form, the composition of the magma changes, following with minerals rich in sodium potassium and aluminum.

Precipitation � The water in Earth’s lakes, rivers, ponds, oceans, and beneath it’s surface contains many dissolved substances. � When this water evaporates, some of the dissolved substances can react to form minerals � Changes in water temperature may also cause dissolved materials to precipitate out of a body of water.

Pressure and Temperature � Some minerals form when existing minerals are subjected to changes in pressure and temperature. � An increase in pressure can cause minerals to recrystallize while under pressure. � The atoms are simply rearranged to form more compact minerals. � Changes in temperature can also cause some minerals to become unstable. � Under these conditions, new minerals form which are stable at the new temperature.

Hydrothermal Solutions � A hydrothermal solution is a very hot mixture of water (between 100 c and 300 c) and dissolved substances. � When these solutions come into contact with existing minerals, chemical reactions take place to form new minerals. � Also, when these solutions cool, some of the elements in them combine to form minerals such as quartz and pyrite.

Mineral Groups � Common minerals, together with the thousands of others that form on Earth, can be classified into groups based on their composition � There are six main mineral groups we will be studying 1. Silicates 2. Carbonates 3. Oxides 4. Sulfates and sulfides 5. Halides 6. Native elements

Silicates � The two most abundant elements in Earth’s crust are silicon and oxygen. � Silicon and oxygen combine to form a structure called the silicon-oxygen tetrahedron which is the basis of silicates. � The tetrahedron, which consists of one silicon atom and four oxygen atoms, provides the framework for every silicate mineral

Carbonates � Carbonates are the second most common mineral group. � Carbonates are minerals that contain the elements carbon, oxygen, and one or more other metallic elements. � Calcite is the most common carbonate mineral. Both limestone and marble are rocks composed of carbonate minerals.

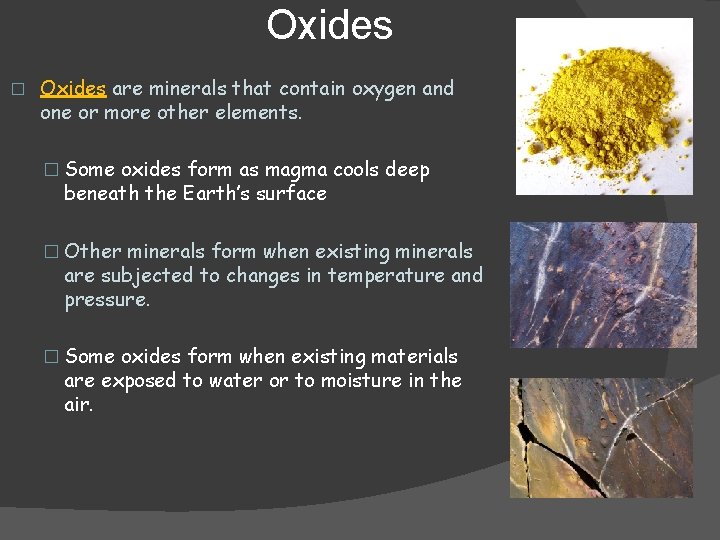
Oxides � Oxides are minerals that contain oxygen and one or more other elements. � Some oxides form as magma cools deep beneath the Earth’s surface � Other minerals form when existing minerals are subjected to changes in temperature and pressure. � Some oxides form when existing materials are exposed to water or to moisture in the air.

Sulfates and Sulfides � Sulfates and sulfides are minerals that contain the element sulfur. � Sulfates form when mineral-rich waters evaporate. � Sulfides often form from thermal, or hot-water solutions.

Halides � Halides are minerals that contain a � halogen ion � plus one or more other elements.

Native Elements � Native Elements are minerals that only contain one element or type of atom. � Elements such as � gold, � silver, � copper, � sulfur, � and carbon are Native Elements

 Most abundant minerals in earth's crust
Most abundant minerals in earth's crust Good earth minerals
Good earth minerals My favourite subject english
My favourite subject english Which weather map symbol is used to represent violently
Which weather map symbol is used to represent violently Earth science sol 2010
Earth science sol 2010 Earth science lab practical
Earth science lab practical Earth science part d
Earth science part d Earth science lab practical
Earth science lab practical Earth science grade 9
Earth science grade 9 Dynamic equilibrium earth science
Dynamic equilibrium earth science Which is part of the hydrosphere?
Which is part of the hydrosphere? Science jeopardy 8th grade
Science jeopardy 8th grade Geology earth science definition
Geology earth science definition Environmental science final
Environmental science final Earth science meaning
Earth science meaning Astronomy definition earth science
Astronomy definition earth science Earth science sol review
Earth science sol review Earth science vs geology
Earth science vs geology Earth science final exam
Earth science final exam Earth science lab practical
Earth science lab practical Earth science semester 2 final exam answers
Earth science semester 2 final exam answers Properties of minerals streak
Properties of minerals streak Air pressure definition earth science
Air pressure definition earth science Earth science reference table dew point
Earth science reference table dew point Earth science branches
Earth science branches