Earth Materials Minerals There are roughly 3000 minerals






















- Slides: 24

Earth Materials: Minerals

• There are roughly 3000 minerals in the crust (lithosphere) but only a handful are commonly found! • Minerals can be made of one element, monomineralic (ex: Iron- Fe) or a polymineralic meaning a compound ( ex: Si. O -quartz) 2 • A Mineral: is a naturally occurring, inorganic (not made by living things), homogenous


How are Minerals formed? • 1. ) Cooling and solidification of molten rock: atoms arrange themselves as magma/lava cools Examples: quartz, muscovite, feldspars • 2. ) Re-crystalization: Atoms rearrange themselves in already existing minerals when there is an increase in temperature and/or pressure. examples: graphite turns to diamond! • 3. ) Precipitation : mineral comes out of solution in supersaturated water with dissolved elements evaporation of saltwater from oceans/lake beds form halite (called“evaporites”) • Calcite deposits form from supersaturated water in oceans and in caves

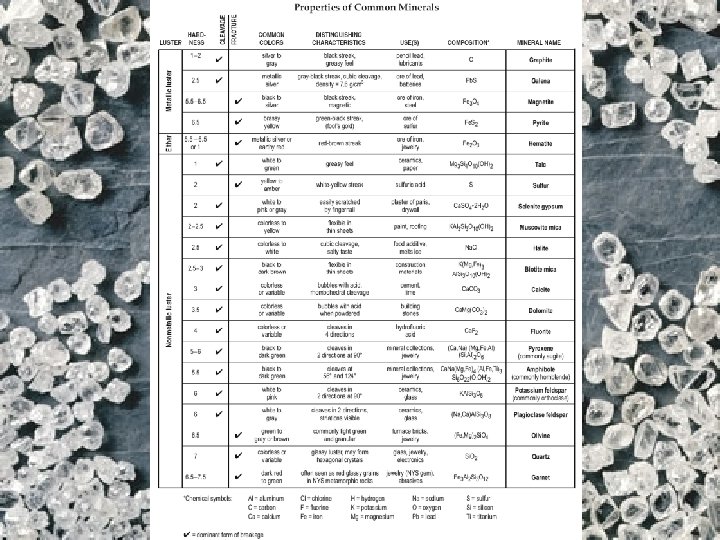
• Minerals are identified by: 1. ) Chemical Properties/Composition: What the mineral is made of (example: Graphite is made of ____) 2. ) Physical Properties : Color, hardness, shape, streak, breakage, etc. *A minerals physical properties are always determined by the internal arrangement of the atoms (how they are organized)

Minerals can have the same chemical ingredients, yet be different in physical properties! Ex: Diamond vs. Graphite

Physical Characteristics For Mineral Identification

Col or Fe Air, Water, CO 2 Pure Si. O 2 • The appearance of the mineral However there are issues with using a color for identification: • FLAW #1 – different minerals can have the same color • FLAW #2 – the same mineral can be different colors! • Variations in color caused by impurities

Luster- the way light is reflected from the surface (NOT HOW SHINY IT IS!) (1)Metallic= looks like metal (2)Non-metallic – Waxy – Pearly, translucent – Earthy /Dull – Glassy/Vitreous

Crystal • Shape/Habit is not always shown Habit/Shape by every mineral because minerals can grow in imperfect conditions. This means tight spaces underground and perhaps not enough time to grow! Halite (Na. Cl)=cubic Calcite (Ca. CO )= 2 rhombohedr on

• Fluorite=octahedron crystal habit • Quartz: Prismatic crystal habit • Garnet: • Octahedral

Streak Pyrite Vs. Gold: Was one of the ways for testing if it was true gold during the gold rush • The color of a mineral’s powder left behind after scratching a “streak plate” (unglazed porcelain tile)

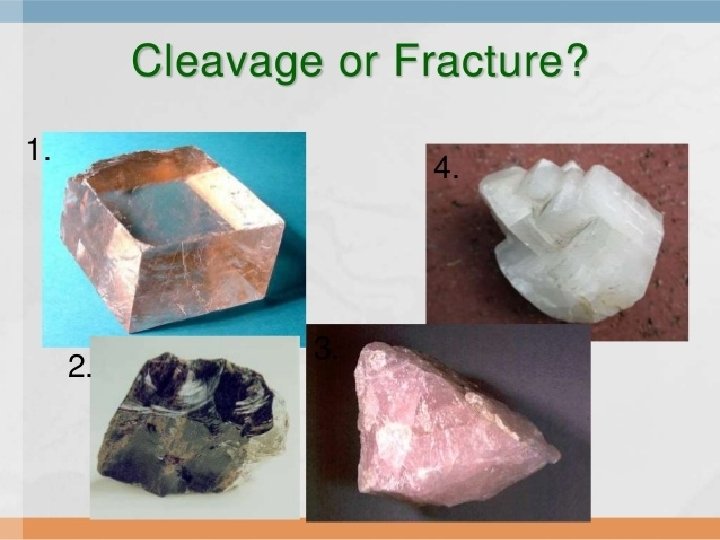
Mineral Breakage: always look on the edges of minerals to distinguish this property! • Cleavage – If a mineral breaks along smooth, even surfaces • Fracture – If it breaks with uneven or jagged surfaces (bumpy)



Hardness (most reliant) • A mineral’s resistance to being scratched • On a scale from 1 -10 • A mineral is said to be hard if it scratches glass (hardness equal or greater than 5) • A mineral is said to be soft if it can not scratch glass

Other Mineral Properties & Identifying Characteristics

acid test • Certain minerals react (bubble) with hydrochloric acid (HCl) – A great way for identifying CALCITE!

Special properties of some minerals • Certain minerals are easily identified by special properties – Fluorite: glows under a black light – Sulfur: bright yellow color, rotten egg smell – Calcite: double refraction, reacts with HCl – Halite: tastes like salt

Mineral Groups 1. ) Silicates • Minerals containing OXYGEN & SILICON – the 2 most abundant elements in the crust • These atoms combine to form the silicaoxygen tetrahedron (Si. O 4) • Look in your ESRT pg. 16 to list all silicate minerals found!

2. )Iron Oxides • Certain minerals contain large amount of IRON (Fe) • When these minerals combine with Oxygen, the result is IRON OXIDE (rust) Ex: Hematite,

3. ) Carbonates • Minerals containing a metallic element and a Carbonate compound CO 3 • Ex: Calcite, Ca. CO 3 • Found in Limestone and Marble • These minerals break down with acid rain and acidic ground water

Your ESRT pg 16 lists all the mineral information you will need to identify an unknown mineral sample! Q: Overall, what DETERMINES a mineral’s

Common Uses of Minerals Garnet - jewelry (NYS gem), abrasives Halite - food additive, melts ice Garnet, North Creek, NY Quartz - glass, jewelry, electronics, abrasives Calcite - cement, toothpaste Gypsum- Drywall, cement, plaster of paris
 Antigentest åre
Antigentest åre 3000+3000+2000
3000+3000+2000 Titanides
Titanides Characteristics of natural approach
Characteristics of natural approach Radio layer in bluetooth
Radio layer in bluetooth Roughly tuned input
Roughly tuned input Background issues in language learning
Background issues in language learning Vedas
Vedas Roughly tuned input
Roughly tuned input Materials or substances such as minerals forests
Materials or substances such as minerals forests Most abundant minerals in earth's crust
Most abundant minerals in earth's crust Good earth minerals
Good earth minerals Favourite cars
Favourite cars Properties of matter useful and harmful materials
Properties of matter useful and harmful materials Man made materials
Man made materials Adopting and adapting teaching materials
Adopting and adapting teaching materials Direct materials budget with multiple materials
Direct materials budget with multiple materials The outer core is made up of
The outer core is made up of Mr lee layers of the earth
Mr lee layers of the earth How much water is there on earth
How much water is there on earth Geographic terms jeopardy
Geographic terms jeopardy Tema there is there are
Tema there is there are What part of speech is open
What part of speech is open There is there are negative form
There is there are negative form There is there are countable uncountable nouns
There is there are countable uncountable nouns