Earth as a System A view of Earth



















- Slides: 39

Earth as a System

A view of Earth n Scientists and others view the Earth as a system of interconnected spheres, not a collection of unrelated parts

What is a System? n. A system is a part of the universe that can be studied separately n In an open system the surroundings freely exchange matter and energy n In a closed system, energy may enter, but matter doesn’t leave

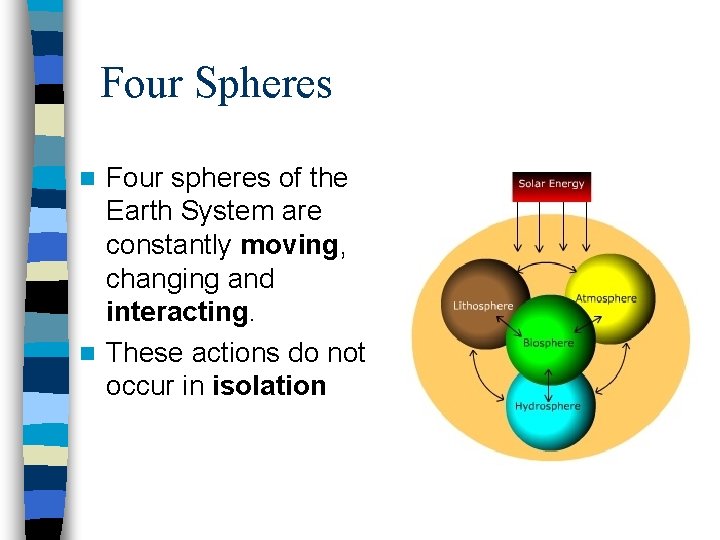
n The Earth is unique in the Solar System; it is the only planet that can support life. Earth is able to do this by the interactions of four spheres Atmosphere, Geosphere, Hydrosphere, and Biosphere

ATMOSPHERE

ATMOSPHERE The gaseous envelope that surrounds the Earthcomposed of gases that help sustain life n Compositionn • 78% Nitrogen (N 2 ), 21% Oxygen (O 2), 0. 9% Argon (Ar), < 1% Carbon Dioxide (CO 2), < 1% water vapor, and trace elements Other gases absorb and alter rays from the sun n Earth’s atmosphere only one in the solar system that has oxygen n

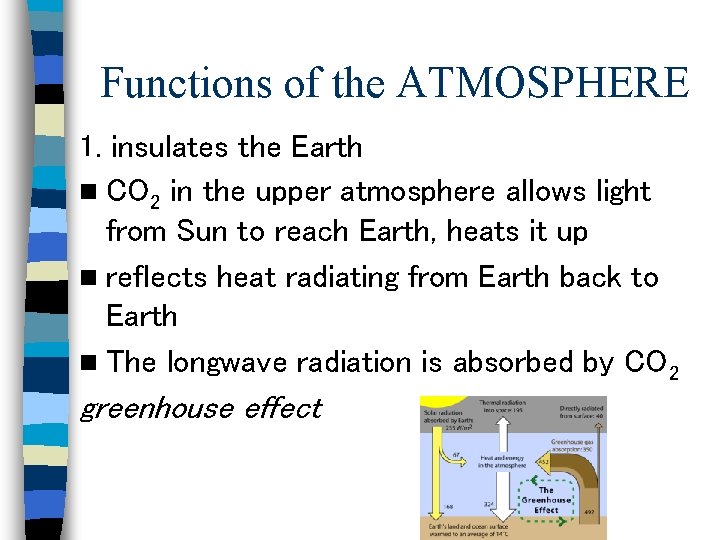
Functions of the ATMOSPHERE 1. insulates the Earth n CO 2 in the upper atmosphere allows light from Sun to reach Earth, heats it up n reflects heat radiating from Earth back to Earth n The longwave radiation is absorbed by CO 2 greenhouse effect

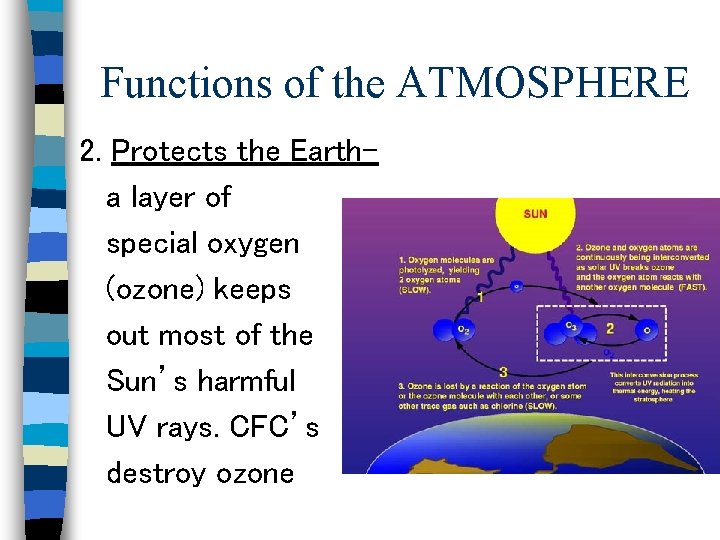
Functions of the ATMOSPHERE 2. Protects the Eartha layer of special oxygen (ozone) keeps out most of the Sun’s harmful UV rays. CFC’s destroy ozone

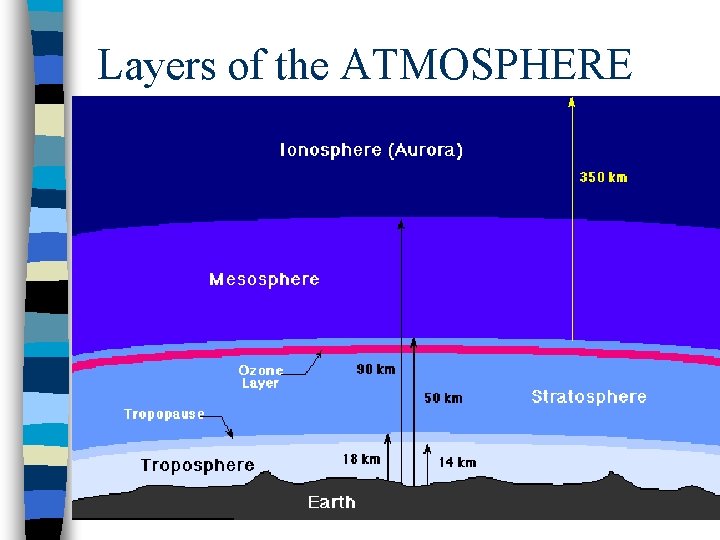
Layers of the ATMOSPHERE

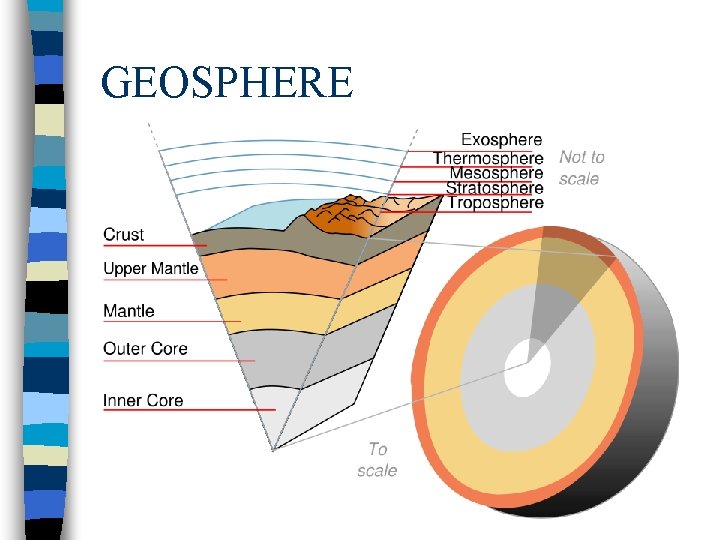
GEOSPHERE n All physical features of the planet except water make up the geosphere n Geosphere changing is always

GEOSPHERE

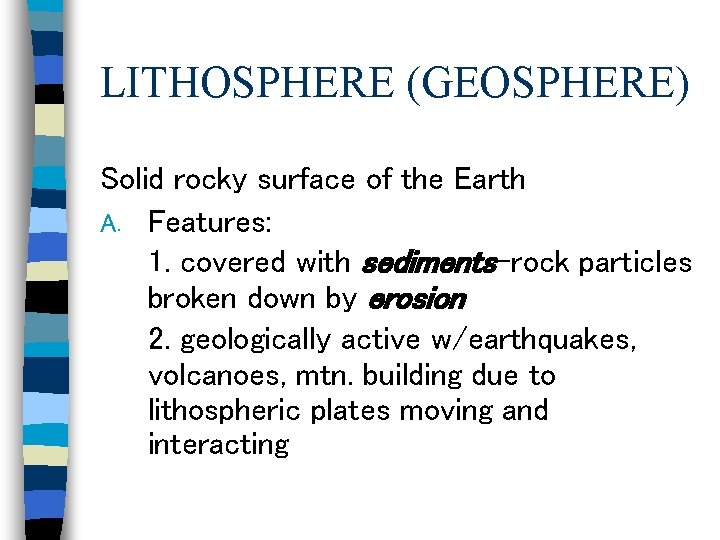
LITHOSPHERE (GEOSPHERE) Solid rocky surface of the Earth A. Features: 1. covered with sediments-rock particles broken down by erosion 2. geologically active w/earthquakes, volcanoes, mtn. building due to lithospheric plates moving and interacting

LITHOSPHERE (GEOSPHERE) B. Functions 1. breaks down rock to provide minerals for soil that make plant life possible 2. provides habitat (swamps, deserts, prairie, etc. ) for tremendously diverse life forms

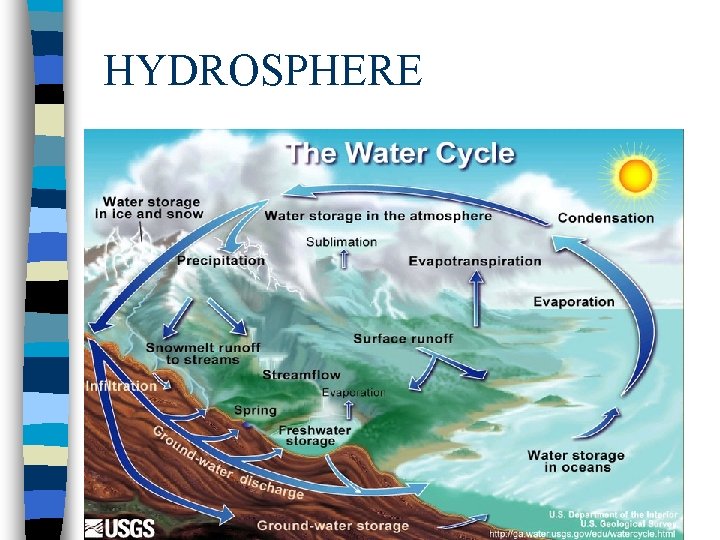
HYDROSPHERE

HYDROSPHERE n n n Contains all the water on Earth including water locked up in ice and snow Earth’s surface Temp’s and Pressure allow H 20 to exist in all 3 states of matter Only 3% is fresh water Covers 70% of Earth’s Surface Water on the Earth is continually recycled

BIOSPHERE n All living organisms on the Earth compose the biosphere n The interactions between the other three spheres gave rise to the conditions that support life

Four Spheres Four spheres of the Earth System are constantly moving, changing and interacting. n These actions do not occur in isolation n

Cycles and the Earth n The water cycle, the carbon cycle, nitrogen cycle and the energy cycle all involve interactions, among the four spheres of Earth. n A cycle is a sequence of events that repeat.

Interactions Change the Spheres n Interactions can take many forms n Single events like a volcano or tornado n Temporary events, such as a heat wave or cold wave n Ongoing, such as erosion

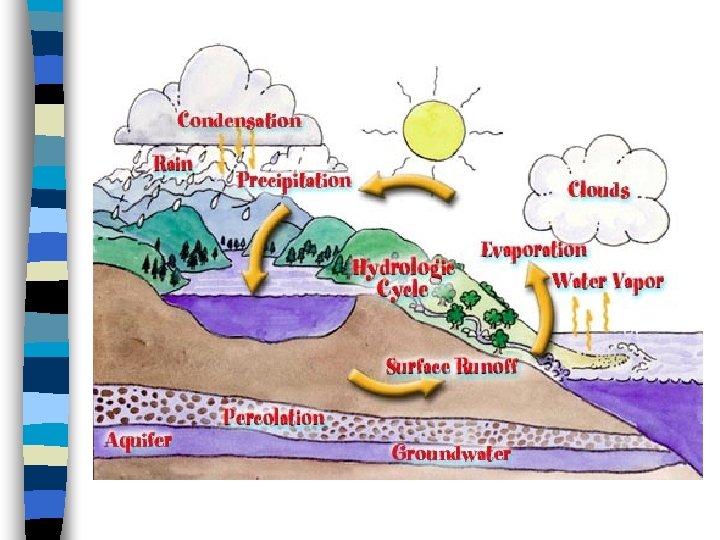
The Water Cycle n Water cycles through the Earth system in solid, liquid and gas forms, but the total amount of water remains relatively constant

Water Cycle n Sun shines on water and causes it to evaporate becoming water vapor, the gaseous state of water n The water vapor rises and cools becoming clouds and forming precipitation. This rain falls down to Earth and fills the rivers, streams etc

Water Cycle cont n Evapotranspiration is the rapid cycling of water vapor into the atmosphere by evaporation from the Earth’s surface or transpiration from plants n When water returns to the oceans, one turn of the water cycle is complete; Water is never created or destroyed only changes “state of matter”


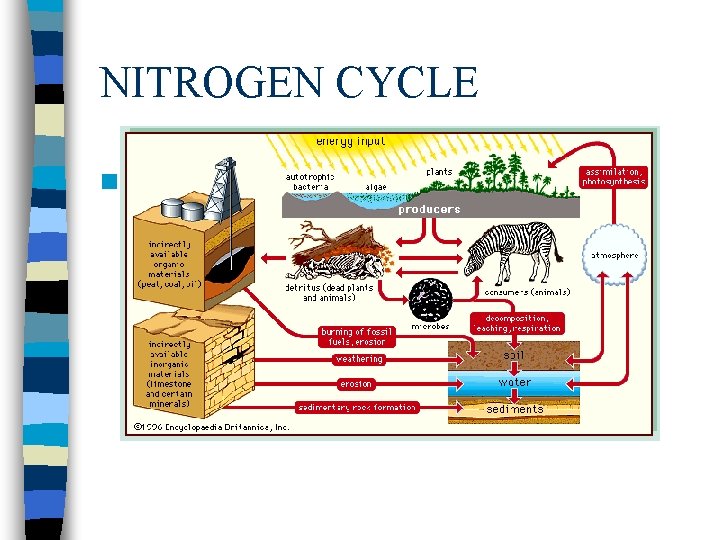
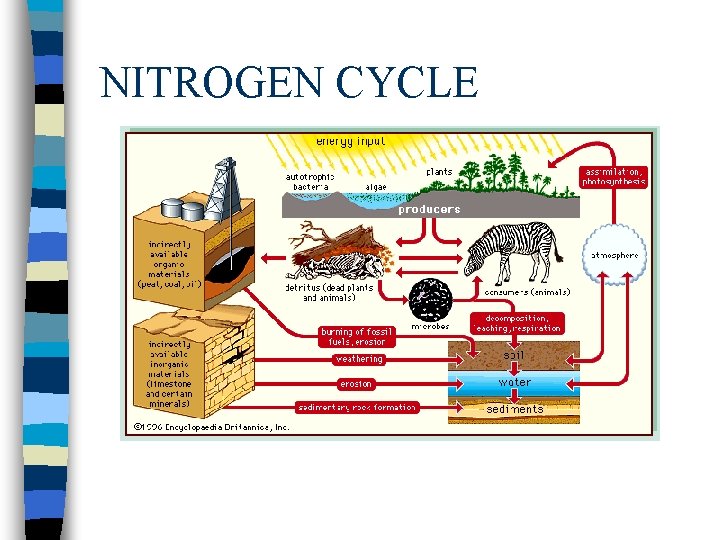
NITROGEN CYCLE n Nitrogen Cycle

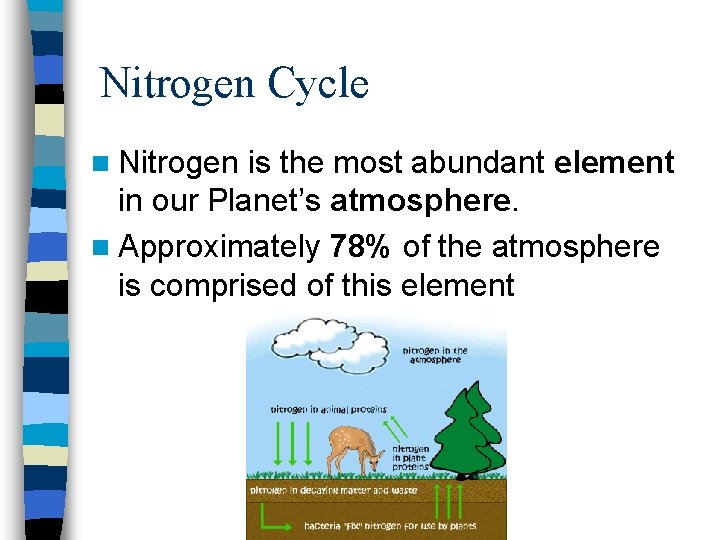
Nitrogen Cycle n Nitrogen is the most abundant element in our Planet’s atmosphere. n Approximately 78% of the atmosphere is comprised of this element

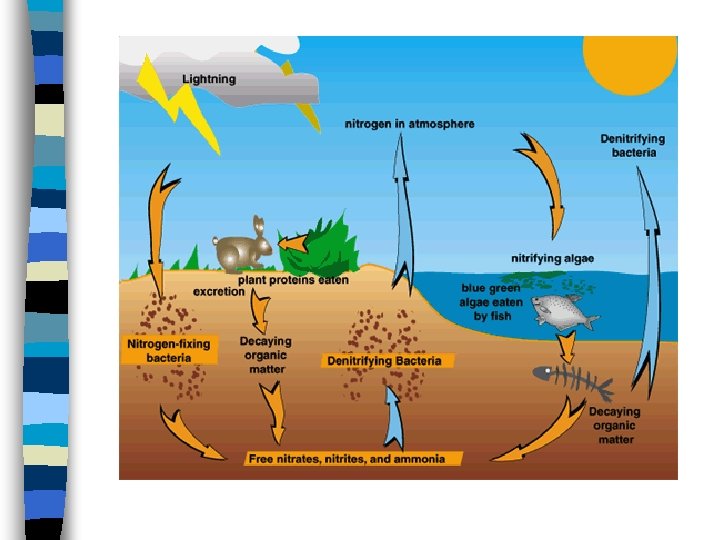
Nitrogen Cycle cont The nitrogen in the air is absorbed by bacteria in the soil or water n The bacteria chemically change nitrogen from the air into nitrogen compounds, which are vital to the growth of plants. n Animals eat the plants absorbing the nitrogen n As animals decay, nitrogen reenter the soil and chemical process release nitrogen back into the atmosphere n

NITROGEN CYCLE


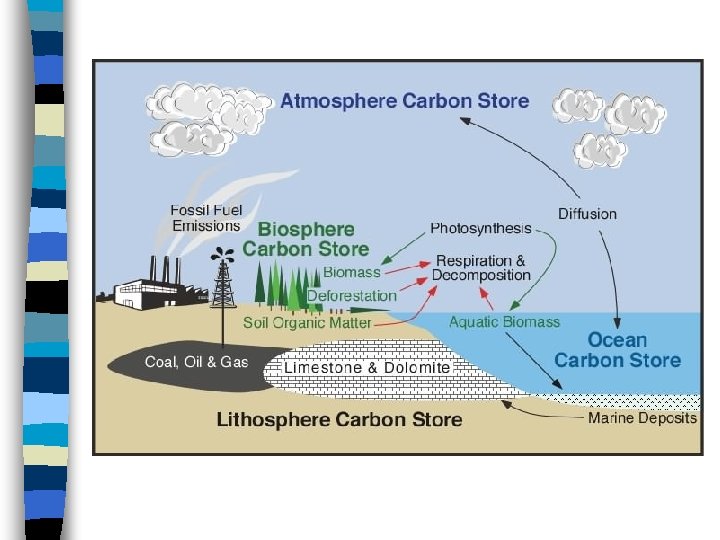
Carbon Cycle n Carbon cycle is another biogeochemical cycle involving the element carbon n Carbon is the building block of life and can be found as a solid or it can form gases n Carbon can be found in all four spheres


Carbon is stored on our planet in the following major sinks : 1) as organic molecules in living and dead organisms found in the biosphere; 2) as the gas carbon dioxide in the atmosphere; 3) in the lithosphere as fossil fuels and sedimentary rock deposits such as limestone, dolomite and chalk; and 4) in the oceans as dissolved atmospheric carbon dioxide and as calcium carbonate shells in marine organisms.

Carbon Cycle n Carbon enters the atmosphere in several ways as CO 2 n Volcanoes add CO 2 n Forest Fires add CO 2

Energy Cycle n If energy in, is greater than energy out climate gets warmer and vice versa n ENERGY – most is from the sun (solar – nuclear fusion) – some is left over from the earth cooling (geothermal – radioactive decay) – The last little bit is from the moon pulling on the oceans (tidal - gravity)

Energy Cycle n Where does the energy go… – – – n Some is reflected Some is used for evaporation and precipitation, Some is converted into wave/wind energy Some is converted into heat energy Some is stored in water, ice, plants and rocks Energy changes and a little bit is lost to the cycle – Lost as heat (the rest of the cycles don’t lose) n ENERGY CAN NEVER BE FULLY RECYCLED!

Energy Cycle n The energy cycle is the movement of energy into and through Earth’s system. n The amount of energy that enters the system should equal the energy that is removed

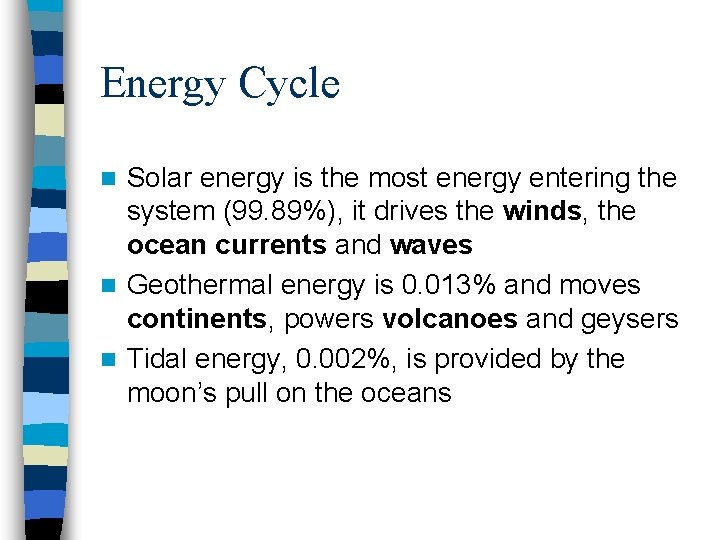
Energy Cycle Solar energy is the most energy entering the system (99. 89%), it drives the winds, the ocean currents and waves n Geothermal energy is 0. 013% and moves continents, powers volcanoes and geysers n Tidal energy, 0. 002%, is provided by the moon’s pull on the oceans n

Law of Thermodynamics Energy follows some basic rules – Thermodynamics is a branch of physics that deals with how heat energy is converted into other forms of energy how energy flows 1 st law states Energy can neither be created nor destroyed n 2 nd law states that when energy changes, it is converted from a more useful, more concentrated form to a less useful, less concentrated form. This means that energy can never be completely recycled n

Effects of the Earth’s Surface n The Earth’s surface is not uniform, it is covered by cities, forests, ocean etc n Different parts of the Earth’s surface reflect solar energy at various rates n The percentage of energy that is reflected without being changed is called albedo

Effects of the Earth’s Surface n Sun reflected differently depending on the surface – Forest absorbs a lot – Snow…. not so much – Desert is in between n How much is reflected can change (seasons, cloud cover, etc. ) n On average the Earth reflects ~30% of the sun’s energy.