EART 160 Planetary Sciences Francis Nimmo Last Week






- Slides: 26

EART 160 Planetary Sciences Francis Nimmo

Last Week • Planetary mass and radius give us bulk density • Bulk density depends on both composition and size • Larger planets have greater bulk densities because materials get denser at high pressures • The increase in density of a material is controlled by its bulk modulus • Planets start out hot (due to accretion) and cool • Cooling is accomplished (usually) by either conduction or convection • Vigour of convection is controlled by the Rayleigh number, and increases as viscosity decreases • Viscosity is temperature-dependent, so planetary temperatures tend to be self-regulating

Talk tomorrow • 4 pm in NS 101 • Matija Cuk, The lunar cataclysm

This Week - Atmospheres • What determines the surface temperature of a planet? • What determines the temperature and pressure structure of planetary atmospheres? • What are the atmospheres made of, and where do they come from? • What determines the wind strengths? • How do planetary atmospheres evolve?

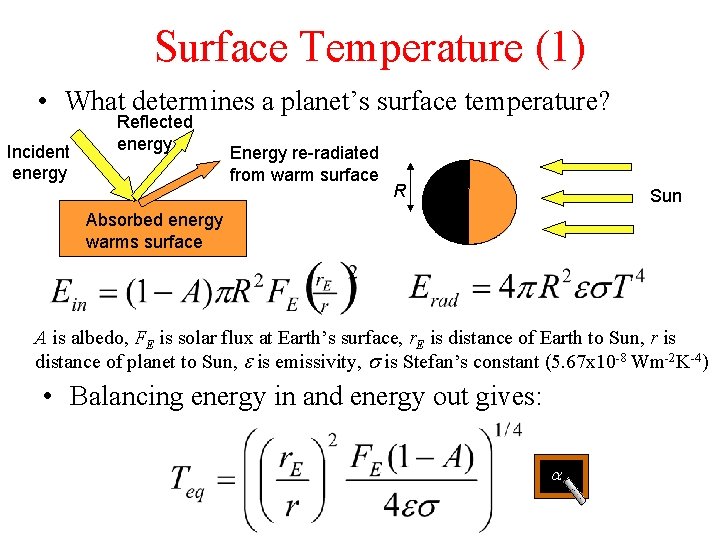
Surface Temperature (1) • What determines a planet’s surface temperature? Incident energy Reflected energy Energy re-radiated from warm surface R Sun Absorbed energy warms surface A is albedo, FE is solar flux at Earth’s surface, r. E is distance of Earth to Sun, r is distance of planet to Sun, e is emissivity, s is Stefan’s constant (5. 67 x 10 -8 Wm-2 K-4) • Balancing energy in and energy out gives: a

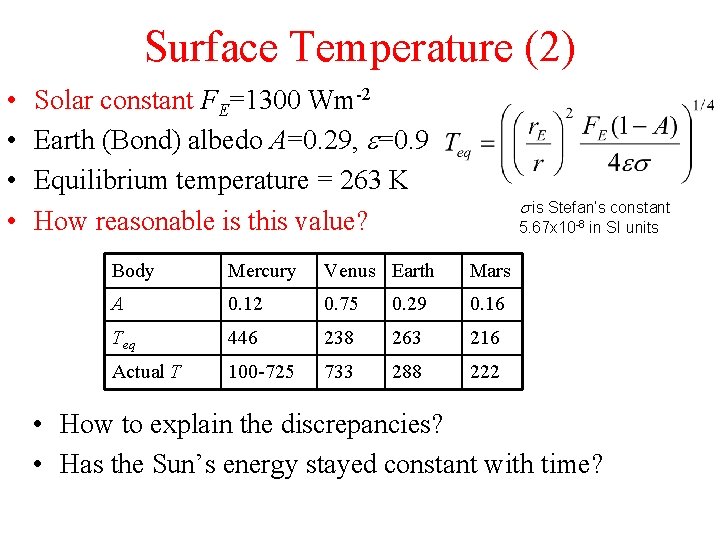
Surface Temperature (2) • • Solar constant FE=1300 Wm-2 Earth (Bond) albedo A=0. 29, e=0. 9 Equilibrium temperature = 263 K How reasonable is this value? s is Stefan’s constant 5. 67 x 10 -8 in SI units Body Mercury Venus Earth Mars A 0. 12 0. 75 0. 29 0. 16 Teq 446 238 263 216 Actual T 100 -725 733 288 222 • How to explain the discrepancies? • Has the Sun’s energy stayed constant with time?

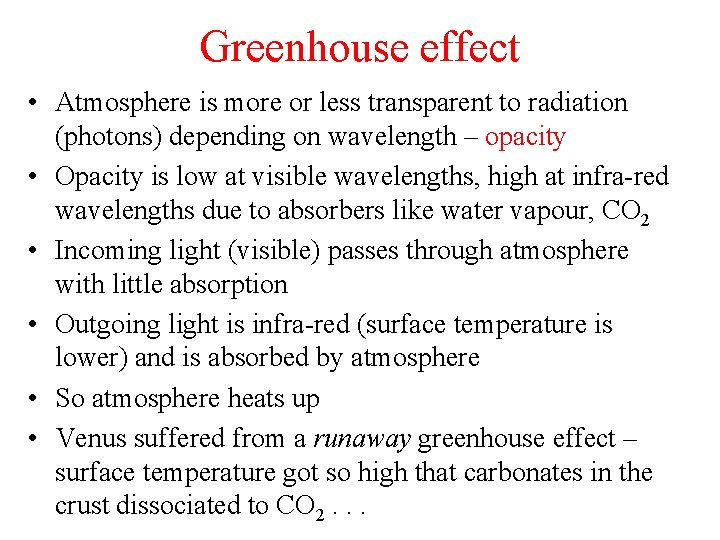
Greenhouse effect • Atmosphere is more or less transparent to radiation (photons) depending on wavelength – opacity • Opacity is low at visible wavelengths, high at infra-red wavelengths due to absorbers like water vapour, CO 2 • Incoming light (visible) passes through atmosphere with little absorption • Outgoing light is infra-red (surface temperature is lower) and is absorbed by atmosphere • So atmosphere heats up • Venus suffered from a runaway greenhouse effect – surface temperature got so high that carbonates in the crust dissociated to CO 2. . .

Albedo effects • Fraction of energy reflected (not absorbed) by surface is given by the albedo A (0<A<1) • Coal dust has a low albedo, ice a high one • The albedo can have an important effect on surface temperature • E. g. ice caps grow, albedo increases, more heat is reflected, surface temperature drops, ice caps grow further. . . runaway effect! • This mechanism is thought to have led to the Proterozoic Snowball Earth • How did the Snowball disappear? • How did life survive? • How might clouds affect planetary albedo?

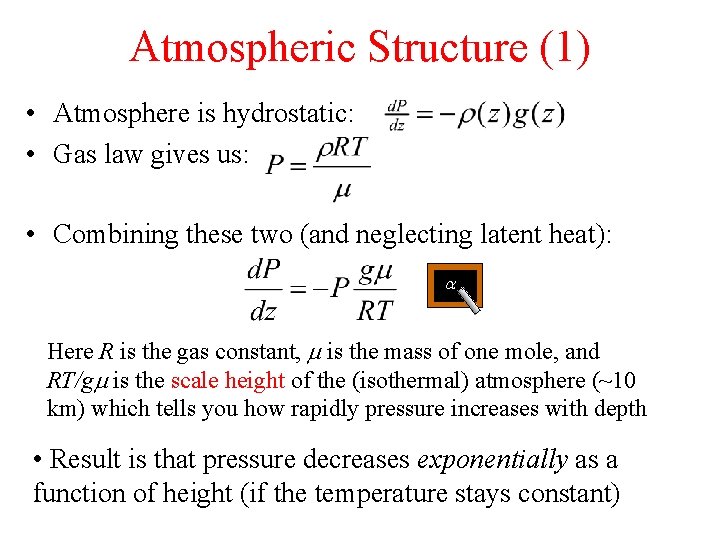
Atmospheric Structure (1) • Atmosphere is hydrostatic: • Gas law gives us: • Combining these two (and neglecting latent heat): a Here R is the gas constant, m is the mass of one mole, and RT/gm is the scale height of the (isothermal) atmosphere (~10 km) which tells you how rapidly pressure increases with depth • Result is that pressure decreases exponentially as a function of height (if the temperature stays constant)

Scale Heights • The scale height tells you how far upwards the atmosphere extends • Scale height H = RT/gm. Does this make physical sense? • Total column mass (per unit area) = r 0 H=P 0/g (where’s this from? ) • It turns out that most planets have similar scale heights: Venus Earth Mars Jupiter Saturn Uranus Neptune Tsurf (K) 733 288 215 165* 135* 76* 72* Albedo 0. 75 0. 29 0. 16 0. 34 0. 29 0. 31 H (km) 16 8. 5 18 18 35 20 19 * Temperature measured at 1 bar pressure

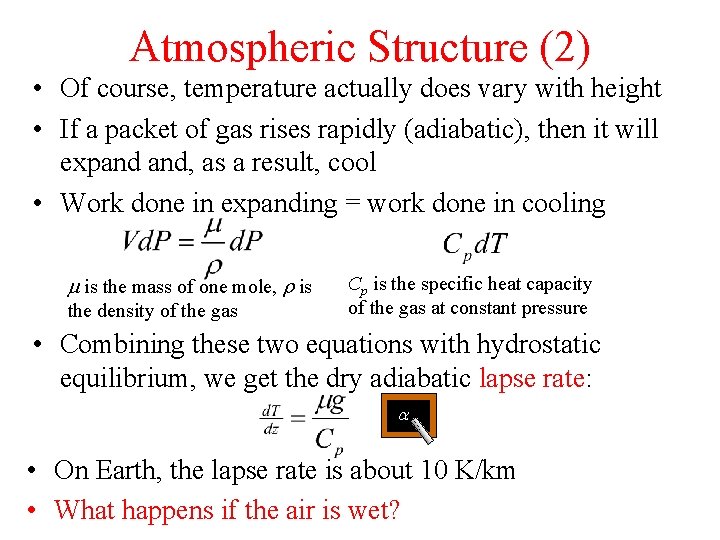
Atmospheric Structure (2) • Of course, temperature actually does vary with height • If a packet of gas rises rapidly (adiabatic), then it will expand and, as a result, cool • Work done in expanding = work done in cooling m is the mass of one mole, r is the density of the gas Cp is the specific heat capacity of the gas at constant pressure • Combining these two equations with hydrostatic equilibrium, we get the dry adiabatic lapse rate: a • On Earth, the lapse rate is about 10 K/km • What happens if the air is wet?

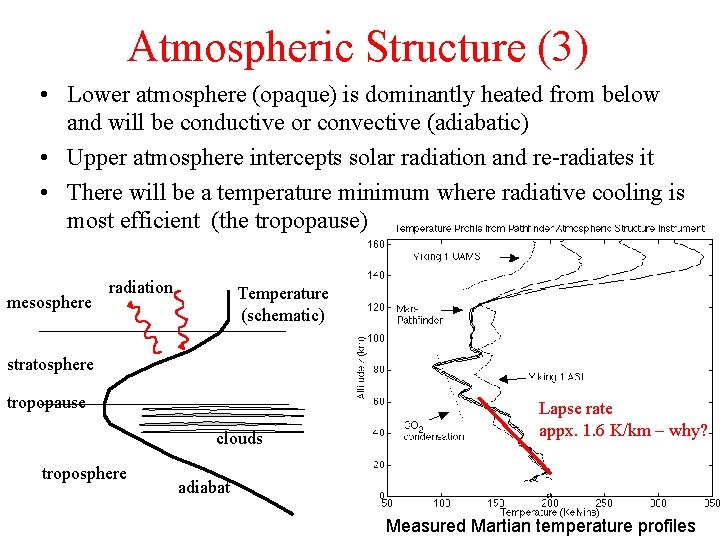
Atmospheric Structure (3) • Lower atmosphere (opaque) is dominantly heated from below and will be conductive or convective (adiabatic) • Upper atmosphere intercepts solar radiation and re-radiates it • There will be a temperature minimum where radiative cooling is most efficient (the tropopause) mesosphere radiation Temperature (schematic) stratosphere tropopause clouds troposphere Lapse rate appx. 1. 6 K/km – why? adiabat Measured Martian temperature profiles

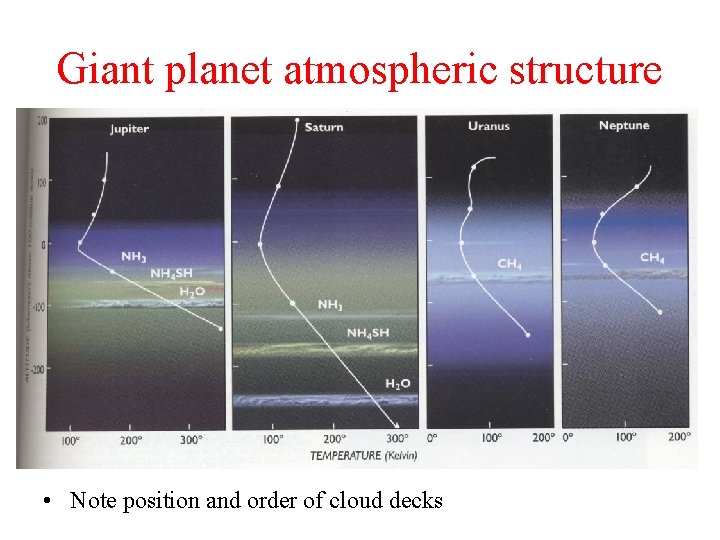
Giant planet atmospheric structure • Note position and order of cloud decks

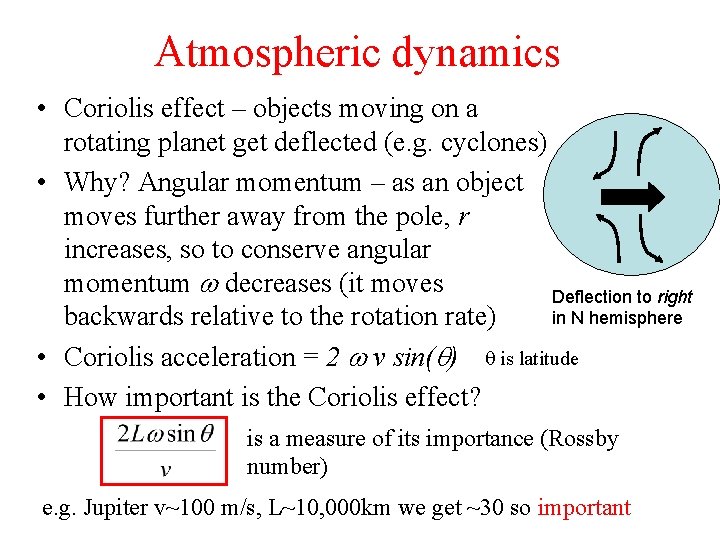
Atmospheric dynamics • Coriolis effect – objects moving on a rotating planet get deflected (e. g. cyclones) • Why? Angular momentum – as an object moves further away from the pole, r increases, so to conserve angular momentum w decreases (it moves Deflection to right in N hemisphere backwards relative to the rotation rate) • Coriolis acceleration = 2 w v sin(q) q is latitude • How important is the Coriolis effect? is a measure of its importance (Rossby number) e. g. Jupiter v~100 m/s, L~10, 000 km we get ~30 so important

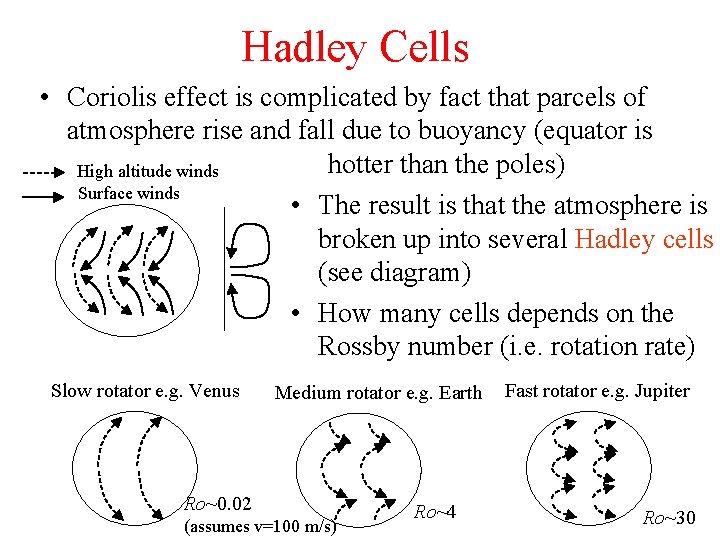
Hadley Cells • Coriolis effect is complicated by fact that parcels of atmosphere rise and fall due to buoyancy (equator is hotter than the poles) High altitude winds Surface winds • The result is that the atmosphere is broken up into several Hadley cells (see diagram) • How many cells depends on the Rossby number (i. e. rotation rate) Slow rotator e. g. Venus Medium rotator e. g. Earth Ro~0. 02 (assumes v=100 m/s) Ro~4 Fast rotator e. g. Jupiter Ro~30

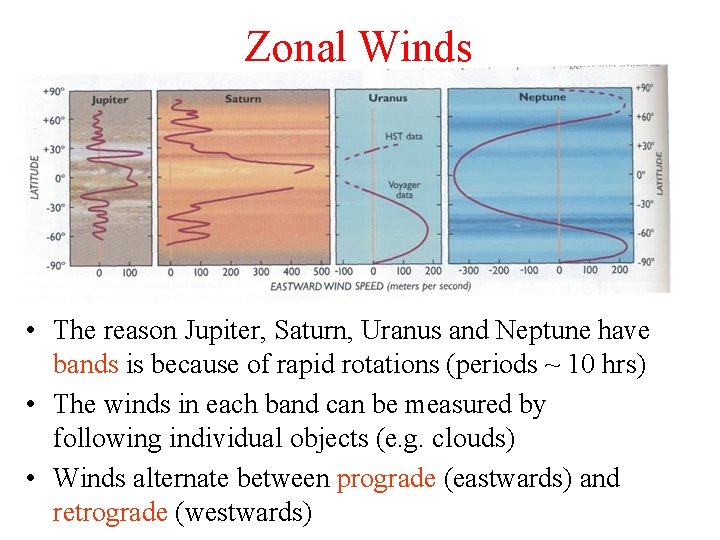
Zonal Winds • The reason Jupiter, Saturn, Uranus and Neptune have bands is because of rapid rotations (periods ~ 10 hrs) • The winds in each band can be measured by following individual objects (e. g. clouds) • Winds alternate between prograde (eastwards) and retrograde (westwards)

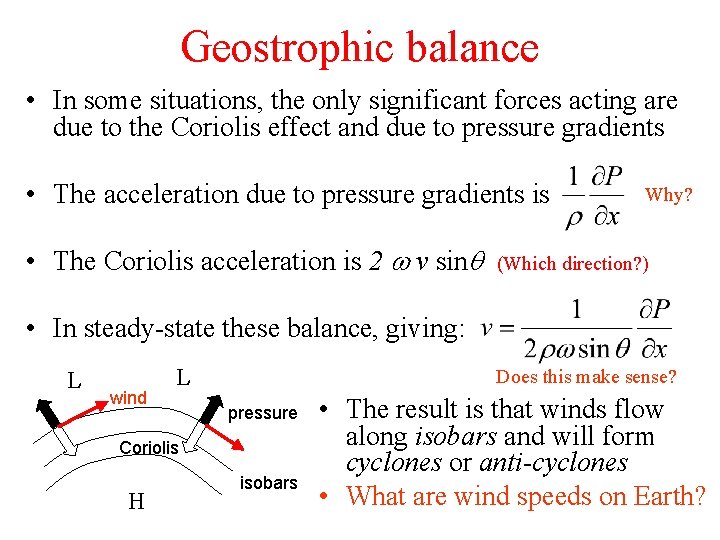
Geostrophic balance • In some situations, the only significant forces acting are due to the Coriolis effect and due to pressure gradients • The acceleration due to pressure gradients is • The Coriolis acceleration is 2 w v sinq Why? (Which direction? ) • In steady-state these balance, giving: L wind L Does this make sense? pressure Coriolis H isobars • The result is that winds flow along isobars and will form cyclones or anti-cyclones • What are wind speeds on Earth?

Where do planetary atmospheres come from? • Three primary sources – Primordial (solar nebula) – Outgassing (trapped gases) – Later delivery (mostly comets) • How can we distinguish these? – Solar nebula composition well known – Noble gases are useful because they don’t react – Isotopic ratios are useful because they may indicate gas loss or source regions (e. g. D/H) – 40 Ar (40 K decay product) is a tracer of outgassing

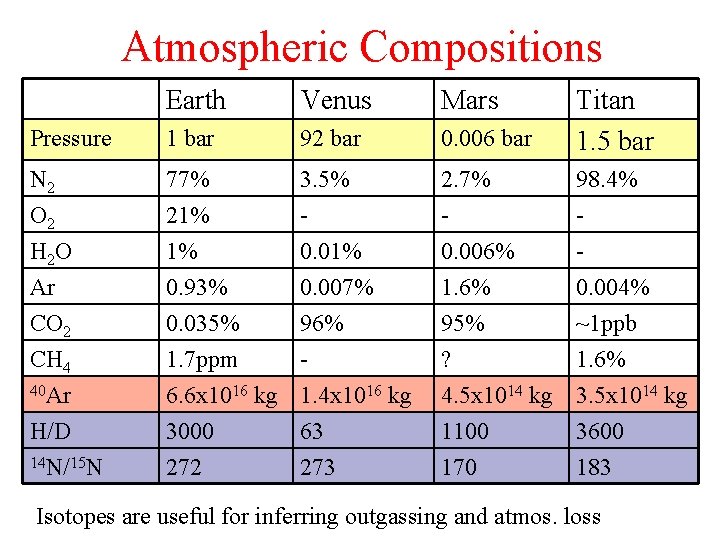
Atmospheric Compositions Earth Venus Mars Pressure 1 bar 92 bar 0. 006 bar Titan 1. 5 bar N 2 O 2 77% 21% 3. 5% - 2. 7% - 98. 4% - H 2 O Ar CO 2 CH 4 40 Ar H/D 14 N/15 N 1% 0. 93% 0. 035% 1. 7 ppm 6. 6 x 1016 kg 3000 272 0. 01% 0. 007% 96% 1. 4 x 1016 kg 63 273 0. 006% 1. 6% 95% ? 4. 5 x 1014 kg 1100 170 0. 004% ~1 ppb 1. 6% 3. 5 x 1014 kg 3600 183 Isotopes are useful for inferring outgassing and atmos. loss

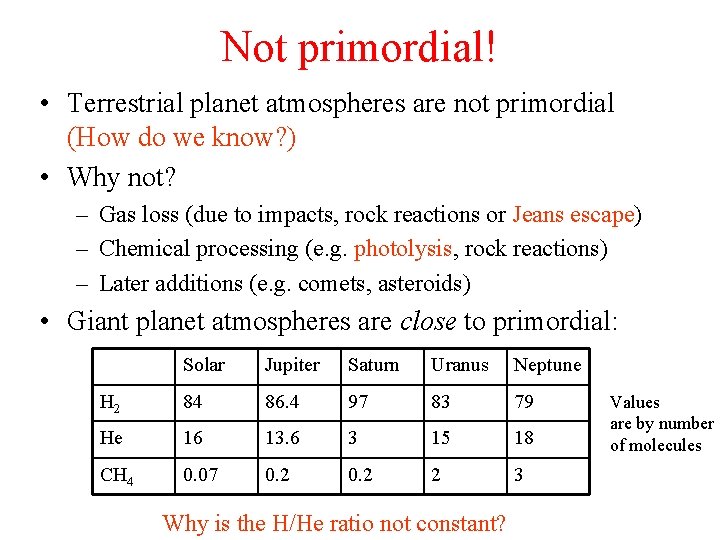
Not primordial! • Terrestrial planet atmospheres are not primordial (How do we know? ) • Why not? – Gas loss (due to impacts, rock reactions or Jeans escape) – Chemical processing (e. g. photolysis, rock reactions) – Later additions (e. g. comets, asteroids) • Giant planet atmospheres are close to primordial: Solar Jupiter Saturn Uranus Neptune H 2 84 86. 4 97 83 79 He 16 13. 6 3 15 18 CH 4 0. 07 0. 2 2 3 Why is the H/He ratio not constant? Values are by number of molecules

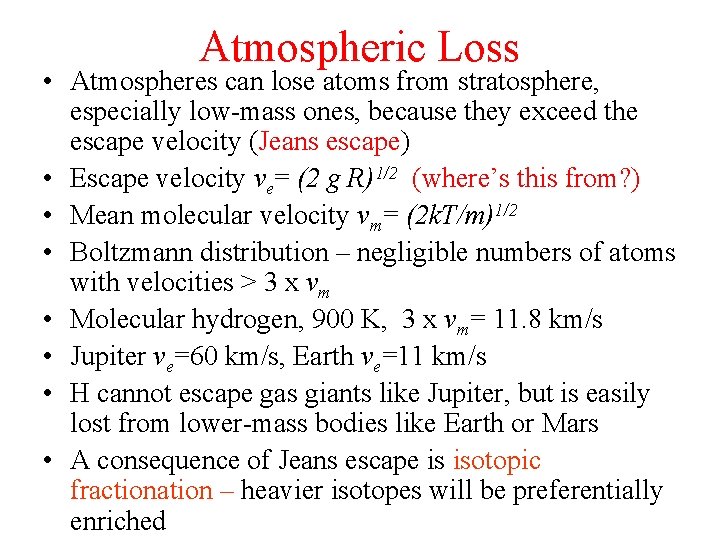
Atmospheric Loss • Atmospheres can lose atoms from stratosphere, especially low-mass ones, because they exceed the escape velocity (Jeans escape) • Escape velocity ve= (2 g R)1/2 (where’s this from? ) • Mean molecular velocity vm= (2 k. T/m)1/2 • Boltzmann distribution – negligible numbers of atoms with velocities > 3 x vm • Molecular hydrogen, 900 K, 3 x vm= 11. 8 km/s • Jupiter ve=60 km/s, Earth ve=11 km/s • H cannot escape gas giants like Jupiter, but is easily lost from lower-mass bodies like Earth or Mars • A consequence of Jeans escape is isotopic fractionation – heavier isotopes will be preferentially enriched

Atmospheric Evolution • Earth atmosphere originally CO 2 -rich, oxygen-free • How do we know? • CO 2 was progressively transferred into rocks by the Urey reaction (takes place in presence of water): • Rise of oxygen began ~2 Gyr ago (photosynthesis & photodissociation) • Venus never underwent similar evolution because no free water present (greenhouse effect, too hot) • Venus and Earth have ~ same total CO 2 abundance • Urey reaction may have occurred on Mars (water present early on), but very little carbonate detected

Summary • Surface temperature depends on solar distance, albedo, atmosphere (greenhouse effect) • Scale height and lapse rate are controlled by bulk properties of atmosphere (and gravity) • Terrestrial planetary atmospheres are not primordial – affected by loss and outgassing • Coriolis effect organizes circulation into “cells” and is responsible for bands seen on giant planets • Isotopic fractionation is a good signal of atmospheric loss due to Jeans escape • Significant volatile quantities may be present in the interiors of terrestrial planets

Key Concepts • • • Albedo and opacity Greenhouse effect Snowball Earth Scale height H = RT/gm Lapse rate Tropopause Coriolis effect 2 w v sin(q) Hadley cell Geostrophic balance Jeans escape Urey reaction Outgassing


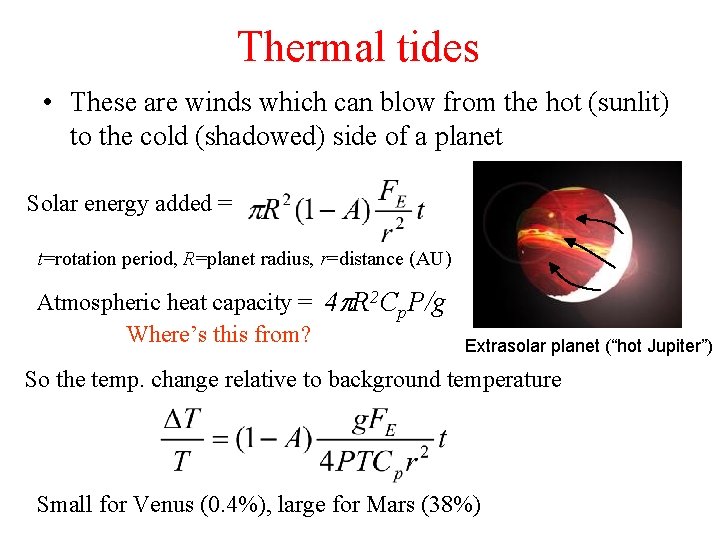
Thermal tides • These are winds which can blow from the hot (sunlit) to the cold (shadowed) side of a planet Solar energy added = t=rotation period, R=planet radius, r=distance (AU) Atmospheric heat capacity = 4 p. R 2 Cp. P/g Where’s this from? Extrasolar planet (“hot Jupiter”) So the temp. change relative to background temperature Small for Venus (0. 4%), large for Mars (38%)