Early History of Atomic Theory Ancient Greece Thales

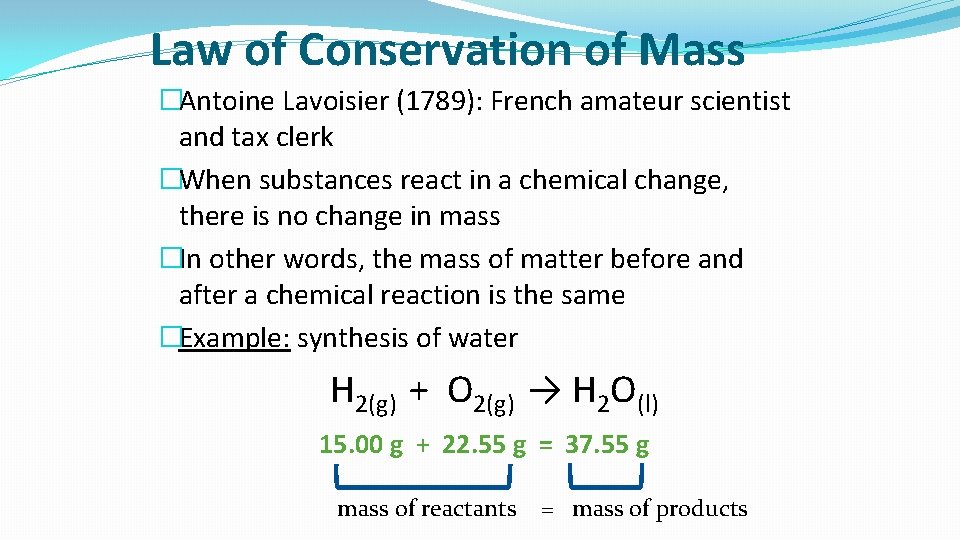

- Slides: 12

Early History of Atomic Theory �Ancient Greece �Thales- Greek philosopher (600 -550 BC) � Smallest particle of all matter was water � First to observe electricity � Autonomous movement interpreted as a “soul” �Empedocles (490 -430 BC) All substances are made of 4 elements � 1. Fire 3. Earth � 2. Air 4. Water � Blend these in different proportions to get all substances � Love and Strife hold matter together

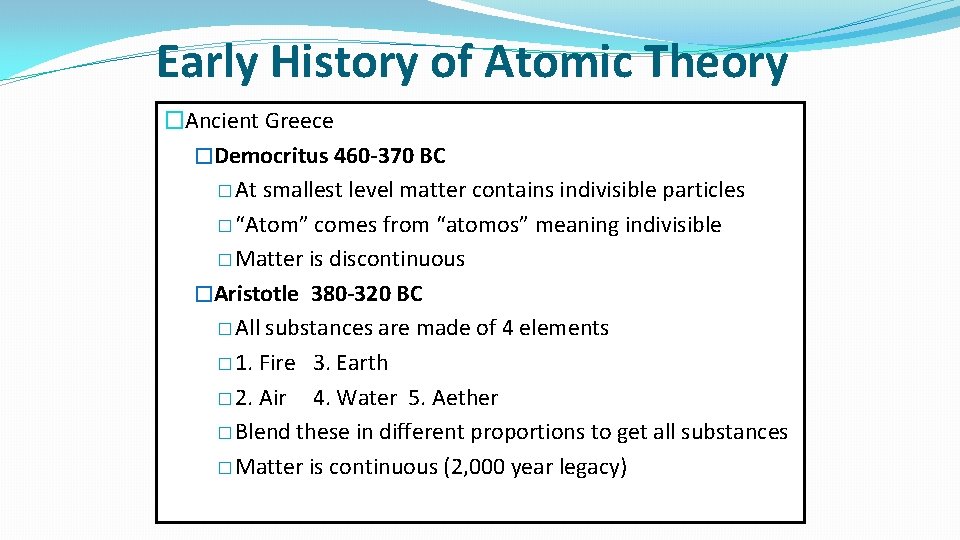
Early History of Atomic Theory �Ancient Greece �Democritus 460 -370 BC � At smallest level matter contains indivisible particles � “Atom” comes from “atomos” meaning indivisible � Matter is discontinuous �Aristotle 380 -320 BC � All substances are made of 4 elements � 1. Fire 3. Earth � 2. Air 4. Water 5. Aether � Blend these in different proportions to get all substances � Matter is continuous (2, 000 year legacy)
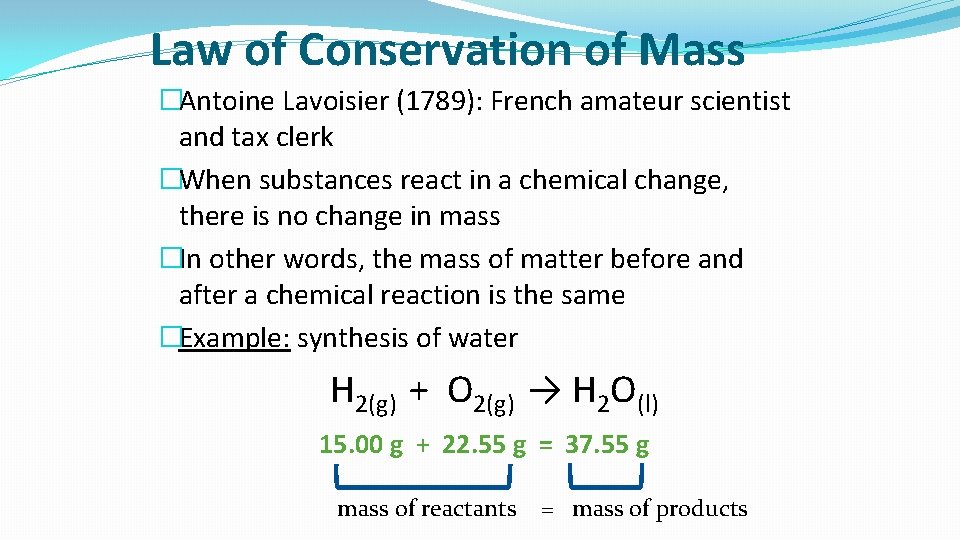
Law of Conservation of Mass �Antoine Lavoisier (1789): French amateur scientist and tax clerk �When substances react in a chemical change, there is no change in mass �In other words, the mass of matter before and after a chemical reaction is the same �Example: synthesis of water H 2(g) + O 2(g) → H 2 O(l) 15. 00 g + 22. 55 g = 37. 55 g mass of reactants = mass of products

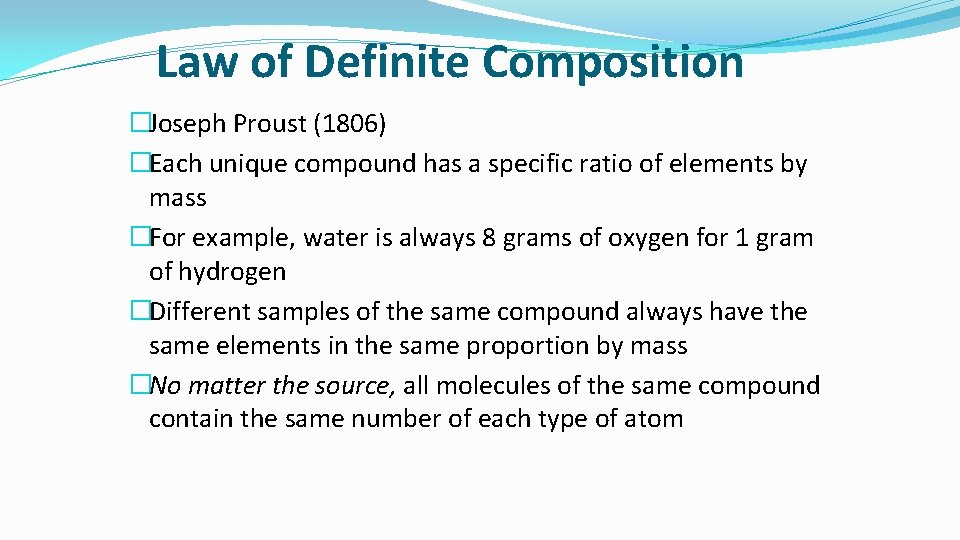
Law of Definite Composition �Joseph Proust (1806) �Each unique compound has a specific ratio of elements by mass �For example, water is always 8 grams of oxygen for 1 gram of hydrogen �Different samples of the same compound always have the same elements in the same proportion by mass �No matter the source, all molecules of the same compound contain the same number of each type of atom

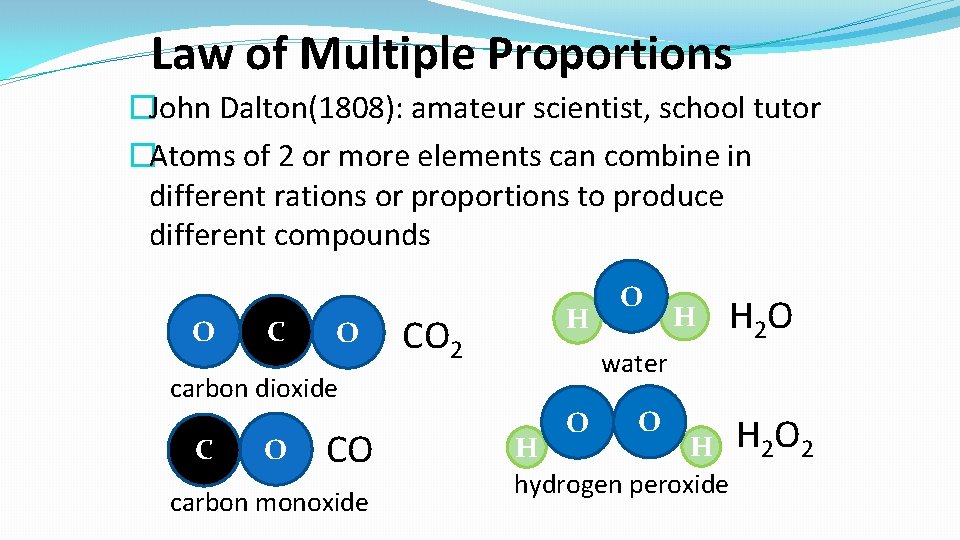
Law of Multiple Proportions �John Dalton(1808): amateur scientist, school tutor �Atoms of 2 or more elements can combine in different rations or proportions to produce different compounds O CO 2 H O CO carbon monoxide H H 2 O water carbon dioxide C O O O H H 2 O 2 H hydrogen peroxide

Dalton’s Atomic Theory (1808) �All matter is made of tiny indivisible particles called atoms. �Atoms of the same element are identical, the atoms of each element are unique. �Atoms of different elements combine in specific whole number ratios to form unique compounds. �Chemical reactions involve the rearrangement of atoms. No new atoms are created or destroyed, no mass gained or lost.

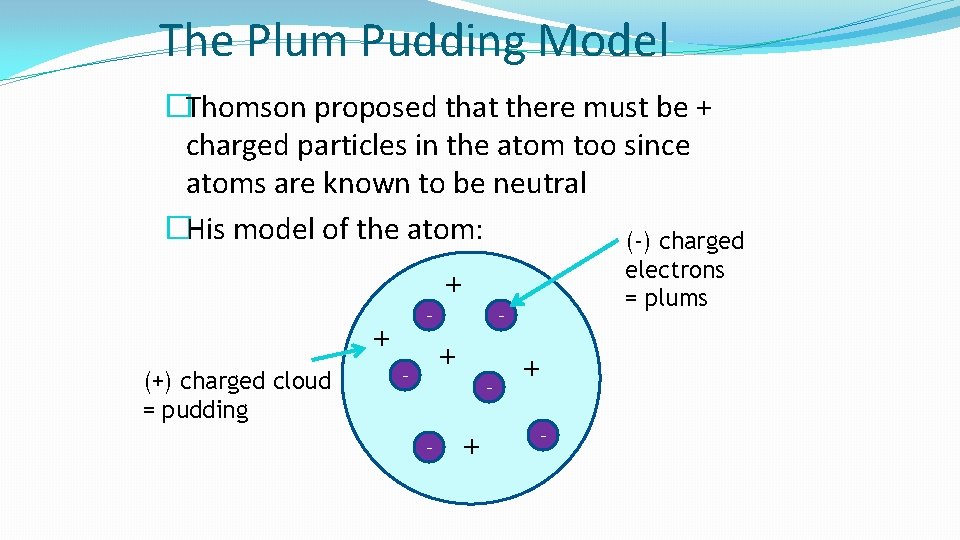
The Plum Pudding Model �Thomson proposed that there must be + charged particles in the atom too since atoms are known to be neutral �His model of the atom: (-) charged electrons = plums + - + (+) charged cloud = pudding - + - - + + -

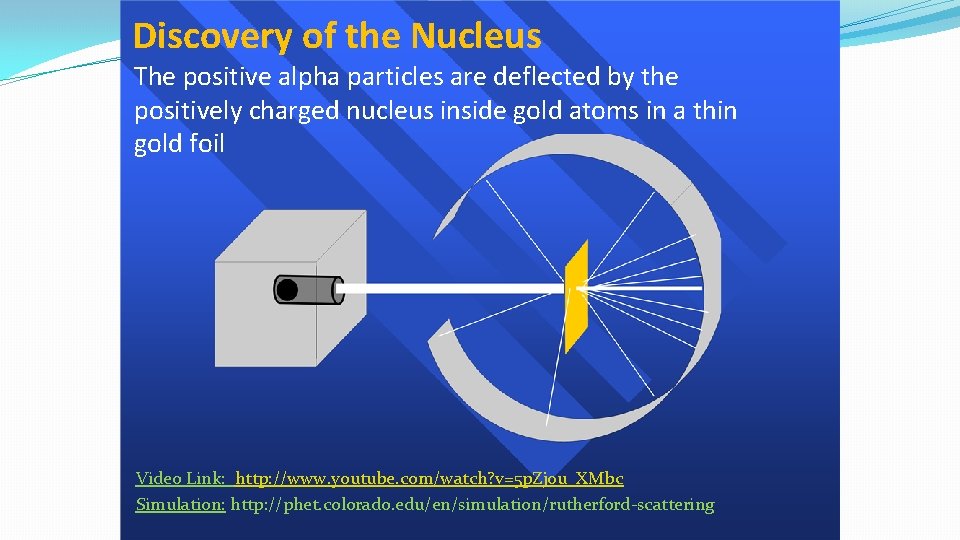
Discovery of the Nucleus The positive alpha particles are deflected by the positively charged nucleus inside gold atoms in a thin gold foil Video Link: http: //www. youtube. com/watch? v=5 p. Zj 0 u_XMbc Simulation: http: //phet. colorado. edu/en/simulation/rutherford-scattering

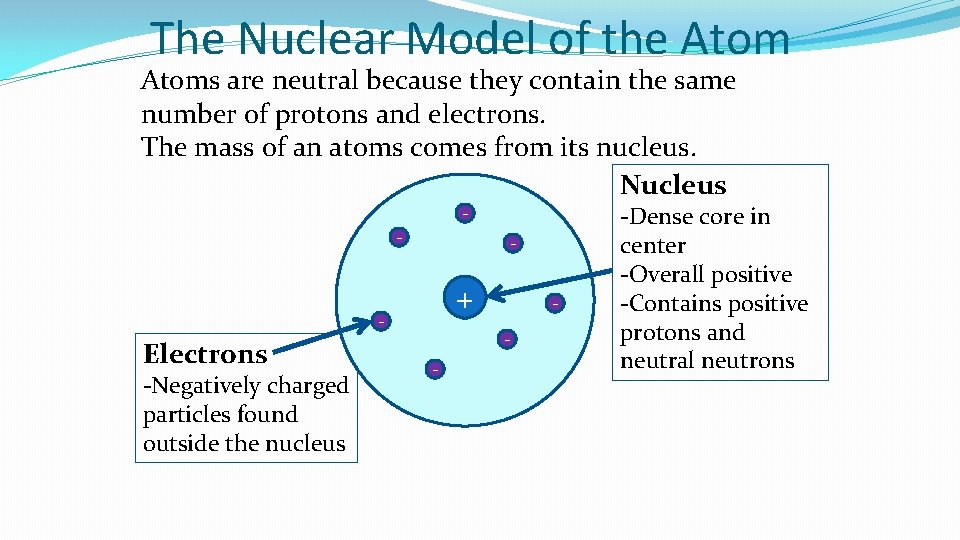
The Nuclear Model of the Atoms are neutral because they contain the same number of protons and electrons. The mass of an atoms comes from its nucleus. Nucleus - - - + - Electrons -Negatively charged particles found outside the nucleus - - -Dense core in center -Overall positive -Contains positive protons and neutral neutrons

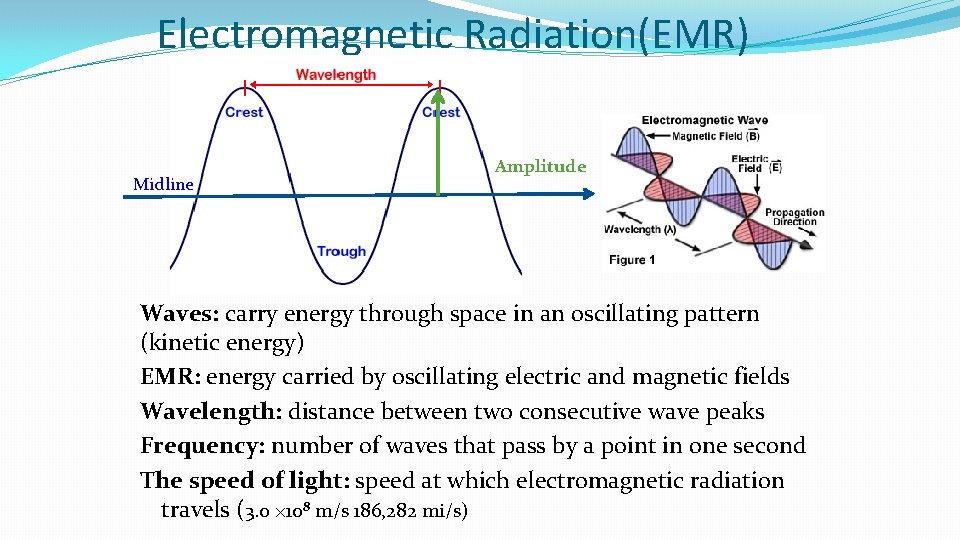
Electromagnetic Radiation(EMR) Midline Amplitude Waves: carry energy through space in an oscillating pattern (kinetic energy) EMR: energy carried by oscillating electric and magnetic fields Wavelength: distance between two consecutive wave peaks Frequency: number of waves that pass by a point in one second The speed of light: speed at which electromagnetic radiation travels (3. 0 × 108 m/s 186, 282 mi/s)

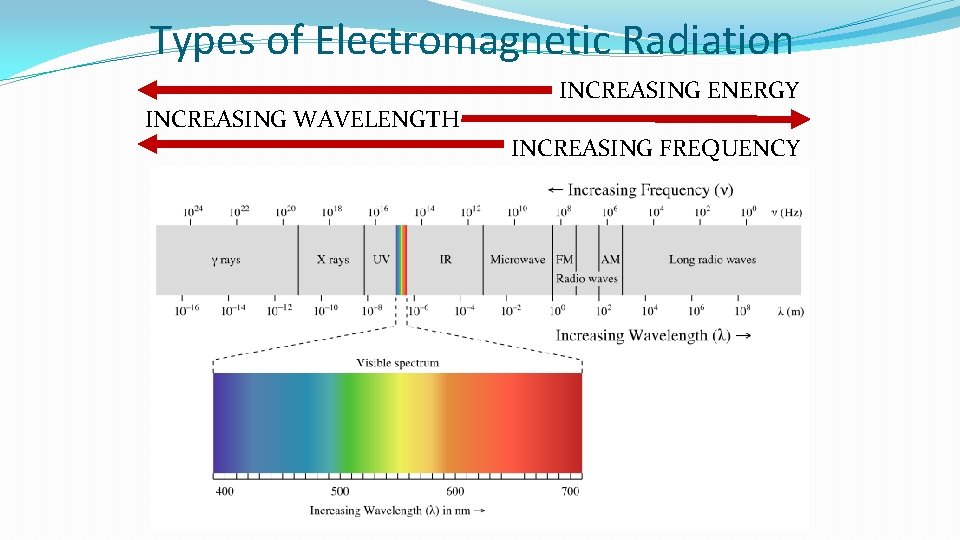
Types of Electromagnetic Radiation INCREASING WAVELENGTH INCREASING ENERGY INCREASING FREQUENCY

Relationships Between Frequency Wavelength and Energy �Frequency and wavelength have an INVERSE relationship �Smaller wavelength = larger frequency �Larger wavelength = smaller frequency �Frequency and energy have a DIRECT relationship �Larger frequency = greater energy �Smaller frequency = less energy �Wavelength and energy have a INVERSE relationship �Smaller wavelength = greater energy � Gamma rays, x-rays, and ultraviolet rays have a small enough wavelength to damage DNA and other important molecules inside cells �Larger wavelength = less energy