Early detection is critical to preserving functional vision


- Slides: 4

Early detection is critical to preserving functional vision, ≥ 20/40 Maintenance of functional (≥ 20/40) vision with Foresee. Home at time of wet AMD diagnosis 1 94% P=. 003 62% Percentage of eyes maintaining 20/40 vision or better With Foresee. Home®, 94% of patients who progressed to wet AMD maintained functional vision (≥ 20/40) vs only 62% of patients using standard detection methods alone 1 Standard care (routine visits + patient reported changes) (n=18) Foresee. Home + standard care (PP 2) (n=29) As few as 17. 5% of patients have a baseline VA of ≥ 20/40 (Snellen equivalent) at treatment initiation based on real-world data. 2 The HOME Study used the ETDRS chart to measure the number of letters for visual acuity. The Snellen equivalent for visual acuity is presented here. Study design: An unmasked, controlled, randomized clinical trial of 1520 participants 53 to 90 years of age with intermediate AMD at high risk of CNV. The study compared home monitoring with Foresee. Home plus standard care vs standard care alone to determine if the addition of the home monitoring device improved visual acuity at the time of CNV detection. 1 CNV=choroidal neovascularization; ETDRS=Early Treatment Diabetic Retinopathy Study; PP 2=per protocol 2 cohort; VA=visual acuity. 1. Chew EY, Clemons TE, Bressler SB, et al; AREDS 2 -HOME Study Research Group. Randomized trial of a home monitoring system 1 | for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014; 121(2): 535 -544. ELEVATING THE CLINICAL BENEFIT 2. De. Croos FC, Reed D, Adam MK, et al. Treat and extend therapy using aflibercept for neovascular age-related macular degeneration: a prospective clinical trial, American Journal of Ophthalmology (2017), doi: 10. 1016/j. ajo. 2017. 06. 002. SM-015. 02

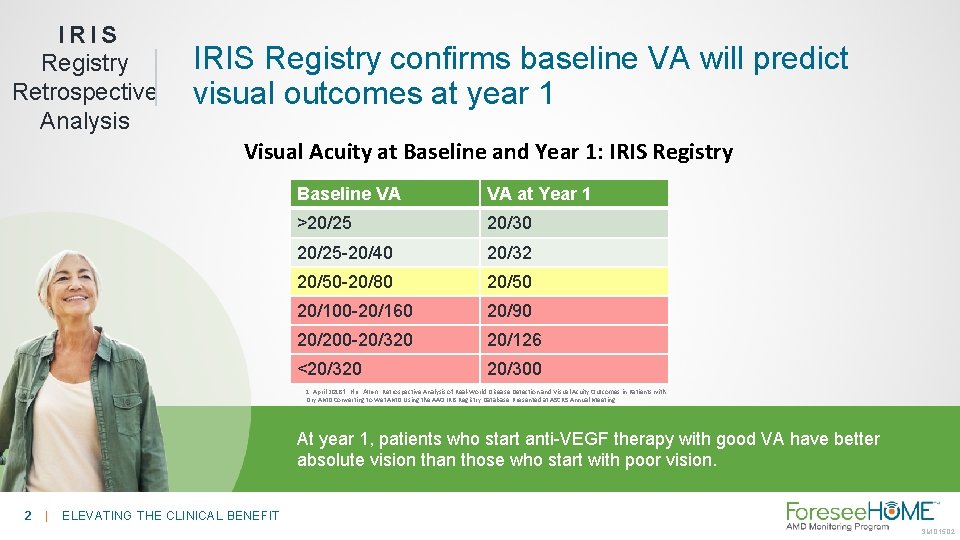
IRIS Registry Retrospective Analysis IRIS Registry confirms baseline VA will predict visual outcomes at year 1 Visual Acuity at Baseline and Year 1: IRIS Registry Baseline VA VA at Year 1 >20/25 20/30 20/25 -20/40 20/32 20/50 -20/80 20/50 20/100 -20/160 20/90 20/200 -20/320 20/126 <20/320 20/300 1. April 20181. Ho, Allen. Retrospective Analysis of Real-World Disease Detection and Visual Acuity Outcomes in Patients with Dry AMD Converting to Wet AMD Using the AAO IRIS Registry Database. Presented at ASCRS Annual Meeting At year 1, patients who start anti-VEGF therapy with good VA have better absolute vision than those who start with poor vision. 2 | ELEVATING THE CLINICAL BENEFIT SM-015. 02

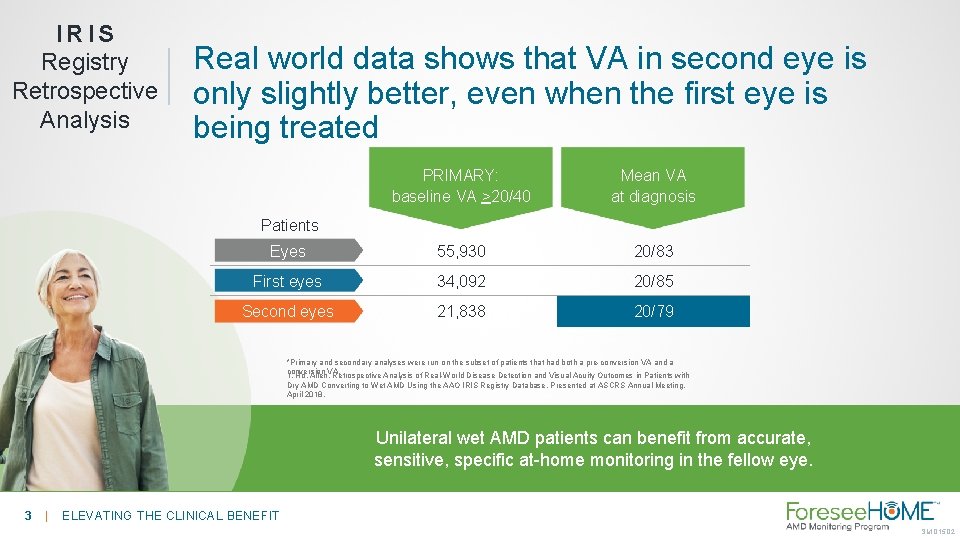
IRIS Registry Retrospective Analysis Real world data shows that VA in second eye is only slightly better, even when the first eye is being treated PRIMARY: baseline VA >20/40 Mean VA at diagnosis Eyes 55, 930 20/83 First eyes 34, 092 20/85 Second eyes 21, 838 20/79 Patients *Primary and secondary analyses were run on the subset of patients that had both a pre-conversion VA and a conversion 1. Ho, Allen. VA. Retrospective Analysis of Real-World Disease Detection and Visual Acuity Outcomes in Patients with Dry AMD Converting to Wet AMD Using the AAO IRIS Registry Database. Presented at ASCRS Annual Meeting. April 2018. Unilateral wet AMD patients can benefit from accurate, sensitive, specific at-home monitoring in the fellow eye. 3 | ELEVATING THE CLINICAL BENEFIT SM-015. 02

Patient | Baseline Vision: 20/20 OD CASE STUDY Patient Case Study • 20/20 at diagnosis, with no change in VA • Patient immediately received anti-VEGF therapy • As of 3/12/18, Patient is 20/20 OD 4 | 20/20 20/40 20/60 20/80 20/100 12/19/16 5/7/17 3/12/18 BASELINE VA 20/20 OD ALERTED AT 20/20 CONTINUES AT 20/20 OD ELEVATING THE CLINICAL BENEFIT SM-015. 02