e Plex Respiratory Pathogen RP Panel Publication Summary

e. Plex® Respiratory Pathogen (RP) Panel Publication Summary CE-IVD. Not available for Sale in the US. GNMK-IMC-2056 -C
Clinical Comparison of e. Plex RP Panel and LDT Real Time PCR 97. 4% concordance with low-plex real time PCR LDT • Strong agreement with real time PCR – 99. 1% agreement when Ct value <35 • Multiple sample types included in study – Nasopharyngeal swab, bronchoalveolar lavage, sputum, throat swab, nasopharyngeal aspirate – 100% concordance with LDT results for non-NPS samples • 15 additional targets detected by the e. Plex RP Panel that were missed by LDT • Short turnaround time and random access are advantageous The e. Plex system provided useful diagnostic data, with minimal hands-on time, and can potentially be used for rapid diagnostic sample-to-answer testing, in either a lab or decentralized setting 1. Nijhuis, R. H. T, et al. 2017. Comparison of e. Plex Respiratory Pathogen Panel with Laboratory Developed Real-Time PCR Assays for Detection of Respiratory Pathogens. J Clin Microbiol 55(6): 1938 -1945. CE-IVD. Not available for Sale in the US. GNMK-IMC-2056 -C

Multicenter Evaluation of the e. Plex RP Panel Rapid and sensitive detection of the most common respiratory pathogens • Rapid diagnosis of respiratory infections can optimize patient care – Improves time to targeted therapy – Can reduce healthcare associated infections with contact and droplet precautions • Performance characteristics evaluated at 13 sites across the US and Canada – Equivalent performance to Bio. Fire overall, with target specific differences – e. Plex RP Panel detected more adenoviruses than Bio. Fire – High agreement for influenza • Hands-on time of 1 min 35 seconds per sample Rapid results and minimal hands-on time of the e. Plex RP Panel enables implementation in larger volume laboratories 1. Babady, N. et al. 2017. Multicenter Evaluation of the e. Plex Respiratory Pathogen Panel for the Detection of Viral and Bacterial Respiratory Tract Pathogens in Nasopharyngeal Swabs. J Clin Microbiol 56(2): e 01658 -17. Published online ahead of print. CE-IVD. Not available for Sale in the US. GNMK-IMC-2056 -C
Clinical Improvements with the e. Plex RP Panel 24 hour reduction in time to result enabled a reduction in isolation time for improved bed management • 85% of patients placed in isolation while waiting for test results – 37% required isolation based on e. Plex test result – ~ half (46%) had isolation discontinued due to e. Plex test result – Median reduction of isolation by 2 days • 1 patient not isolated was determined to require isolation – e. Plex test result prevented 3 days of exposure to influenza A H 3 • Reduction in unnecessary antimicrobial use by ~1 day • 2 patients were positive for bacterial targets not ordered by clinician – B. pertussis and M. pneumoniae Rapid diagnosis promotes more efficient use of isolation rooms, improving bed management during flu season 1. van Rijn, A. , et al. 2017. Clinical Implications of Rapid e. Plex Respiratory Pathogen Panel Testing Compared to Laboratory Developed Real Time PCR. Eur J Clin Microbiol Infect Dis 39&3): 571 -577. CE-IVD. Not available for Sale in the US. GNMK-IMC-2056 -C

A Comparison of Multiplex Respiratory Panels Workflow Analysis • Batched methods are technically complex and have lengthy time-to-result – Limits overall lab efficiency, clinical utility for treatment, bed management, and infection control • Workflow analysis performed on current method vs. rapid, sample-to-answer test – Cepheid Xpert® Flu/RSV with reflex to x. TAG RVP vs. e. Plex RP Panel • Benefits of the e. Plex RP Panel: – – 24/7 testing capability Reduced time-to-result (38 hour decrease) Supports decentralization of testing to individual hospitals Improved utilization of lab space Consistent, reliable result reporting enables providers to optimize care and improve lab efficiency 1. A comparison of multiplex respiratory panels: a workflow analysis. Tingley, L. , et al. CVS 2017. https: //www. genmarkdx. com/resources/publications/ CE-IVD. Not available for Sale in the US. GNMK-IMC-2056 -C
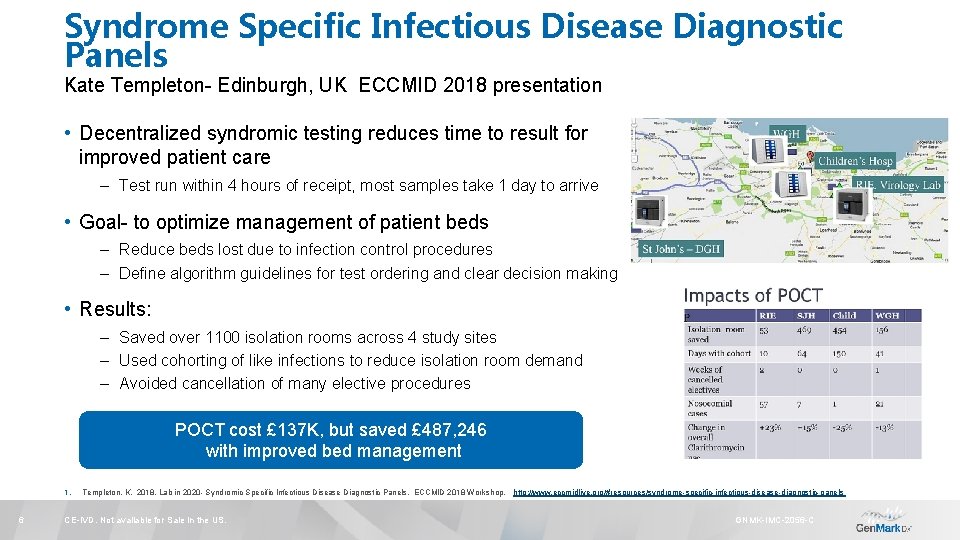
Syndrome Specific Infectious Disease Diagnostic Panels Kate Templeton- Edinburgh, UK ECCMID 2018 presentation • Decentralized syndromic testing reduces time to result for improved patient care – Test run within 4 hours of receipt, most samples take 1 day to arrive • Goal- to optimize management of patient beds – Reduce beds lost due to infection control procedures – Define algorithm guidelines for test ordering and clear decision making • Results: – Saved over 1100 isolation rooms across 4 study sites – Used cohorting of like infections to reduce isolation room demand – Avoided cancellation of many elective procedures POCT cost £ 137 K, but saved £ 487, 246 with improved bed management 1. 6 Templeton, K. 2018. Lab in 2020 - Syndromic Specific Infectious Disease Diagnostic Panels. ECCMID 2018 Workshop. http: //www. eccmidlive. org/#resources/syndrome-specific-infectious-disease-diagnostic-panels CE-IVD. Not available for Sale in the US. GNMK-IMC-2056 -C
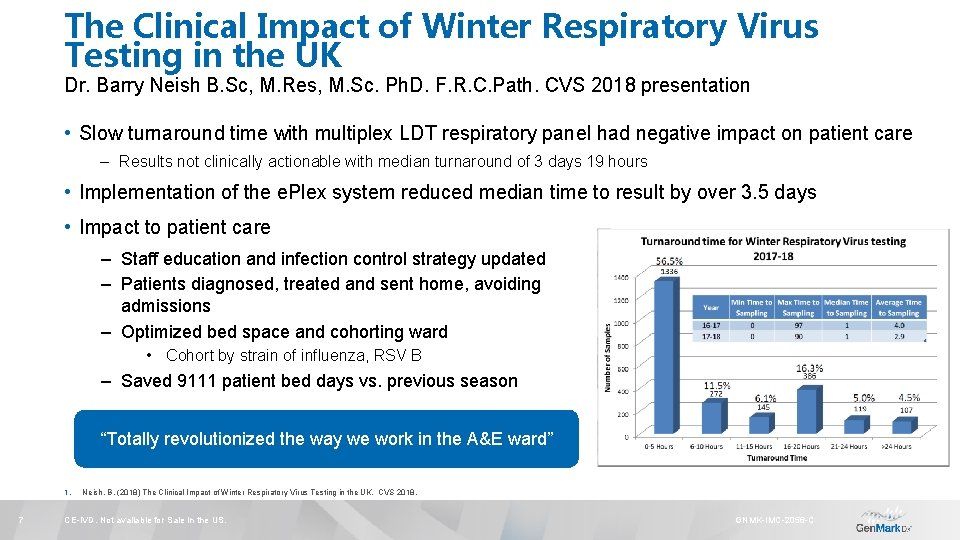
The Clinical Impact of Winter Respiratory Virus Testing in the UK Dr. Barry Neish B. Sc, M. Res, M. Sc. Ph. D. F. R. C. Path. CVS 2018 presentation • Slow turnaround time with multiplex LDT respiratory panel had negative impact on patient care – Results not clinically actionable with median turnaround of 3 days 19 hours • Implementation of the e. Plex system reduced median time to result by over 3. 5 days • Impact to patient care – Staff education and infection control strategy updated – Patients diagnosed, treated and sent home, avoiding admissions – Optimized bed space and cohorting ward • Cohort by strain of influenza, RSV B – Saved 9111 patient bed days vs. previous season “Totally revolutionized the way we work in the A&E ward” 1. 7 Neish, B. (2018) The Clinical Impact of Winter Respiratory Virus Testing in the UK. CVS 2018. CE-IVD. Not available for Sale in the US. GNMK-IMC-2056 -C
Evaluation of a New FDA-Cleared Multiplex PCR Test for Respiratory Pathogens in NPS Dirks, D. C. , et al. University of Virginia, CVS 2018 • e. Plex RP Panel demonstrated 98. 8% concordance in retrospective testing of 74 samples – 1 false negative for PIV 4 – Included fresh and retrospective samples – 8 confirmed co-detections identified, with additional codetections unable to be verified due to sample degradation or freeze/thaw cycling • Based on study results, e. Plex RP Panel was implemented for use in patient testing e. Plex RP Panel as the primary test is appropriate and costeffective when multiplex testing is requested by clinicians 1. 8 Dirks, D. C. , et. al. Evaluation of a New FDA-Cleared Multiplex PCR Test for Respiratory Pathogens in NPS. CVS 2018. CE-IVD. Not available for Sale in the US. GNMK-IMC-2056 -C
- Slides: 8