Dye Colorant which is homogeneously dispersed in the






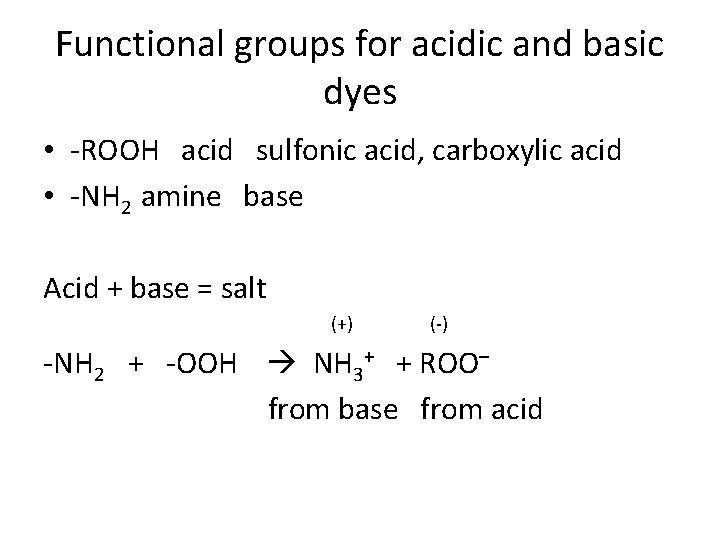


- Slides: 34

Dye: • Colorant which is homogeneously dispersed in the dye medium • Usually soluble • Naturally occurring or synthetic organics

Pazyryk carpet (Siberia) 5 th century BC Peruvian textile 600200 B. C.

Medieval Guilds of Master Dyers • Formed in Germany in the 12 th century • Apprentice system • Dye recipes were heavily guarded secrets

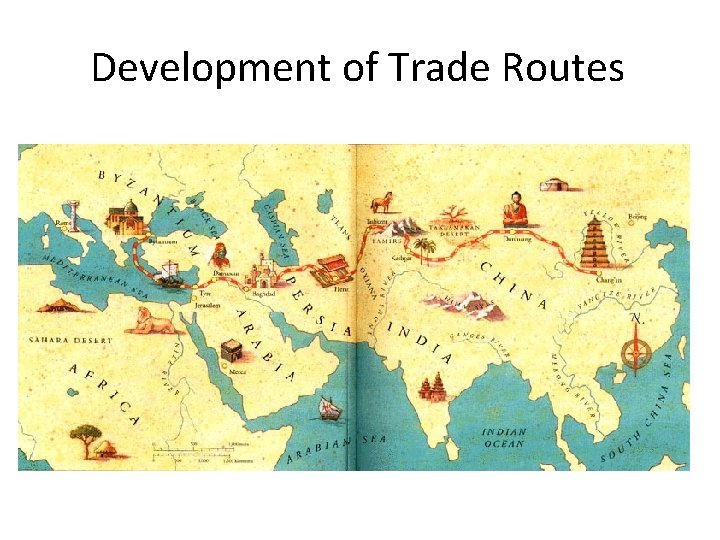
Development of Trade Routes • Textile trade China Europe – beginnings in 2, 000 B. C. – the “Silk Road” – sea routes from the Americas (15 th century) – East India Trading Company (17 th century) • Exchange of not only textiles but also designs, ideas and dyestuffs

Three Classes of Natural Dyes • Substantive dyes – Easiest to apply to fibers – No need to use other substances to enhance dyeing • Vat dyes – Involve chemical change of dye through “fermentation” or “chemical reduction” to make it soluble • Adjective dyes (Mordant dyes) – Require another chemical to develop color and “fix” it to the fiber

Dyes from Snails • Tyrian Purple or “Royal Blue” • 9000 snails to obtain 1 g of dye • Used primarily before 8 th century A. D. to dye wool and silk • Chemically it is 6, 6’dibromoindigo

Dyes from Bugs • Kermes — the most ancient dye in Europe 70, 000 female oak beetles produce 1 pound dye • Cochineal — Mexico and Central America Mexican cactus beetle

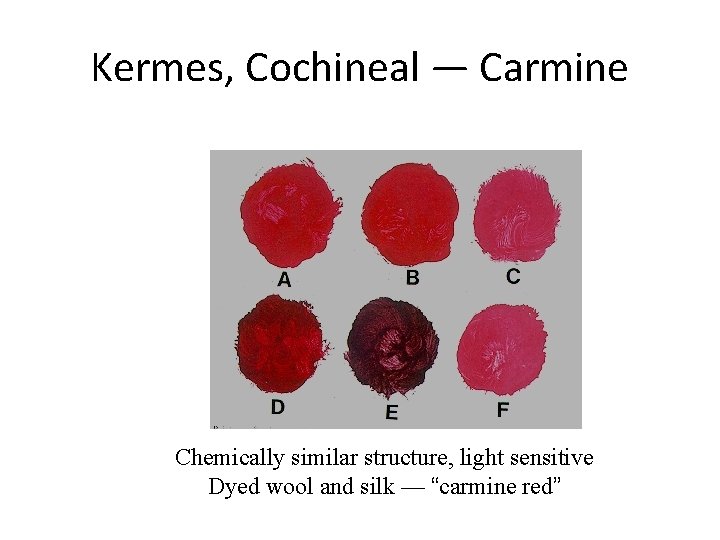
Kermes, Cochineal — Carmine Chemically similar structure, light sensitive Dyed wool and silk — “carmine red”

Dyes From Plants • Indigo — used since 2000 B. C. – – – Extracted from Indigofera tinctoria “Navy Blue” of English sailors Blue jeans Insoluble in water Must be chemical reduced to soluble leucoindigo to use as dye • Woad – Member of the mustard family – A common weed in temperate climates – Leaves contain same chemical as indigo but in lower amounts – Celtic war paint and tattoos Braveheart – Blue robes of priests

More Dyes From Plants • Madder — “Turkey Red” – Root of madder plant found in Europe and Asia – Prepared as a “lake” with Al(OH)3 – British “Redcoats” – Alexander the Great used it to simulate blood Chemically — Alizarin and Pupurin Synthetic alizarin prepared in 1875

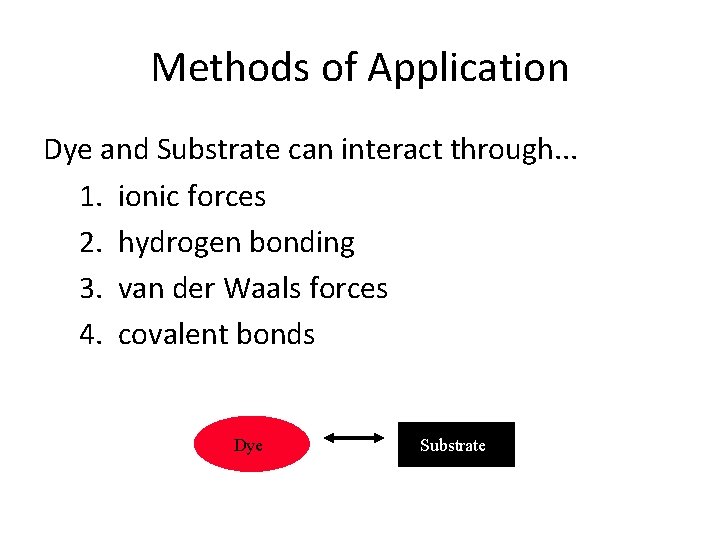
Methods of Application Dye and Substrate can interact through. . . 1. ionic forces 2. hydrogen bonding 3. van der Waals forces 4. covalent bonds Dye Substrate

Types of Dyes by Application • • • Acid Dyes Basic Dyes Mordant Dyes Direct Dyes Fiber-reactive Dyes Vat Dyes
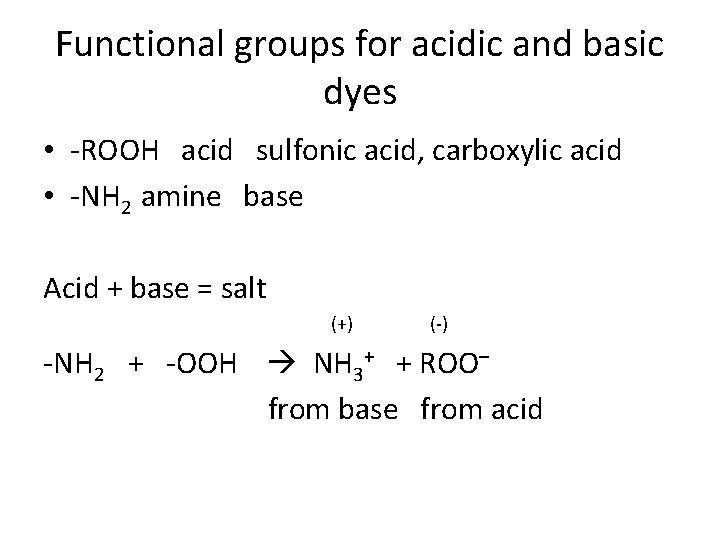
Functional groups for acidic and basic dyes • -ROOH acid sulfonic acid, carboxylic acid • -NH 2 amine base Acid + base = salt (+) (-) -NH 2 + -OOH NH 3+ + ROO– from base from acid

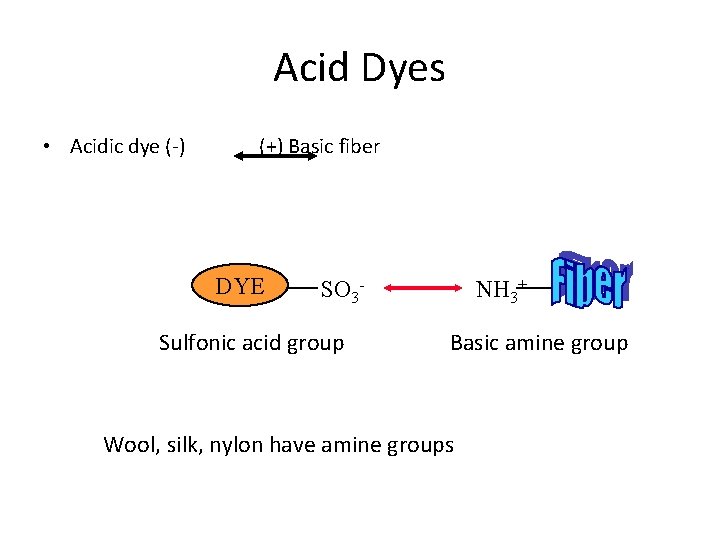
Acid Dyes • Acidic dye (-) (+) Basic fiber DYE NH 3+ SO 3 - Sulfonic acid group Basic amine group Wool, silk, nylon have amine groups

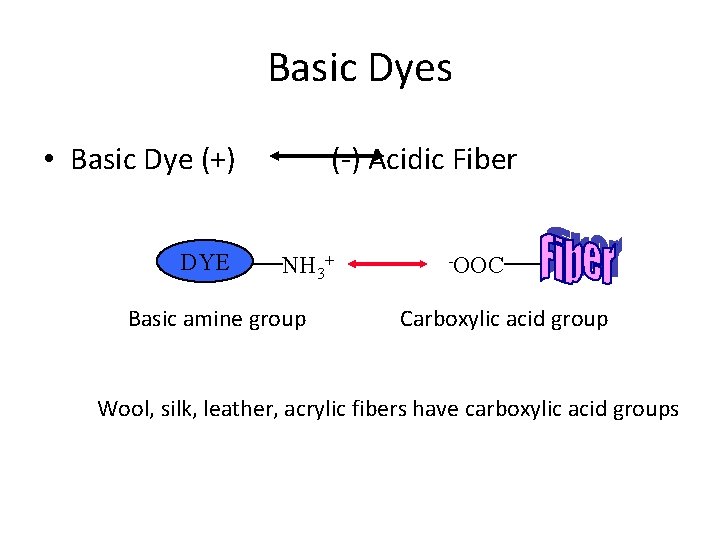
Basic Dyes • Basic Dye (+) DYE (-) Acidic Fiber NH 3+ Basic amine group -OOC Carboxylic acid group Wool, silk, leather, acrylic fibers have carboxylic acid groups

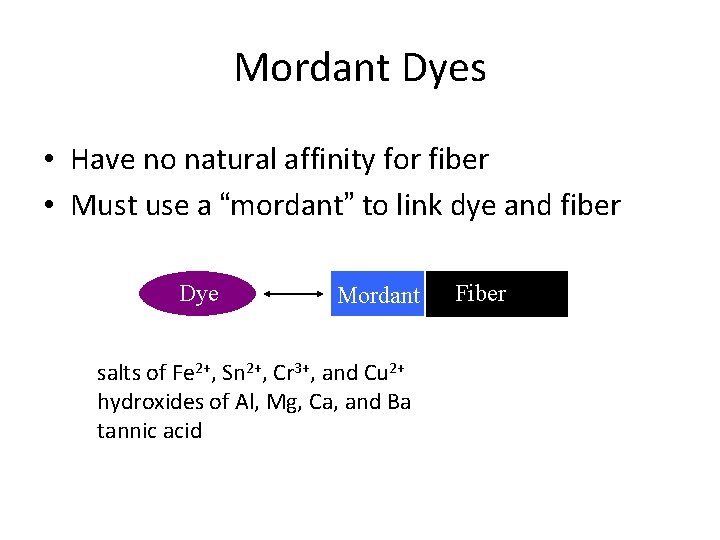
Mordant Dyes • Have no natural affinity for fiber • Must use a “mordant” to link dye and fiber Dye Mordant salts of Fe 2+, Sn 2+, Cr 3+, and Cu 2+ hydroxides of Al, Mg, Ca, and Ba tannic acid Fiber

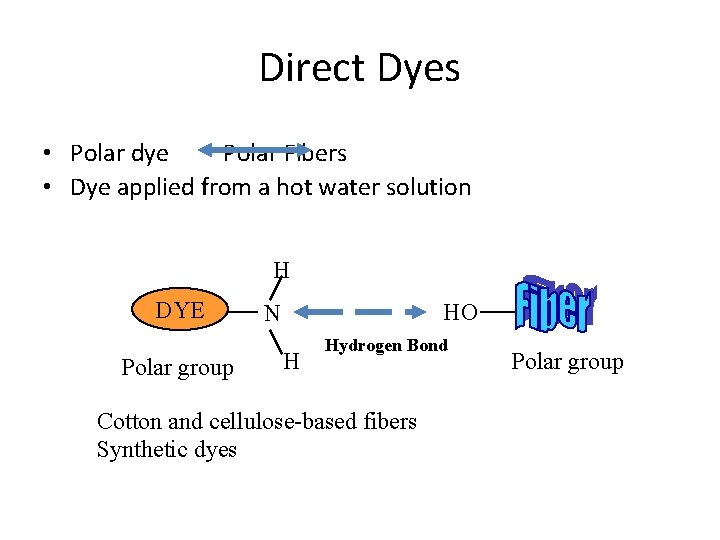
Direct Dyes • Polar dye Polar Fibers • Dye applied from a hot water solution H DYE Polar group HO N H Hydrogen Bond Cotton and cellulose-based fibers Synthetic dyes Polar group

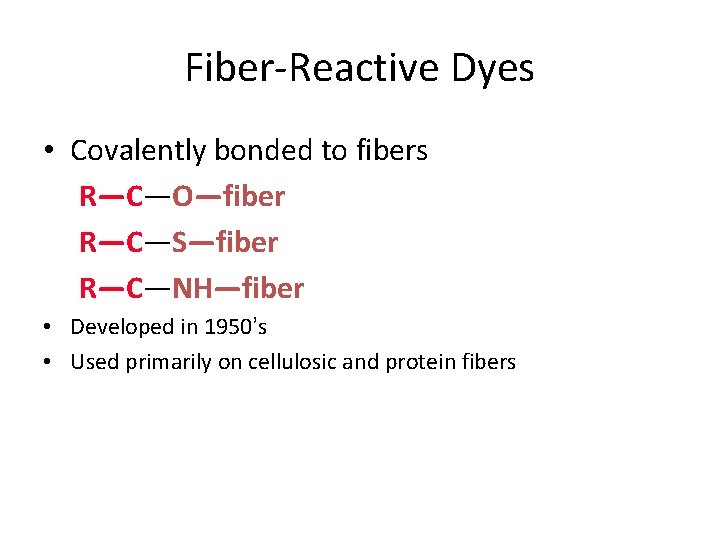
Fiber-Reactive Dyes • Covalently bonded to fibers R—C—O—fiber R—C—S—fiber R—C—NH—fiber • Developed in 1950’s • Used primarily on cellulosic and protein fibers

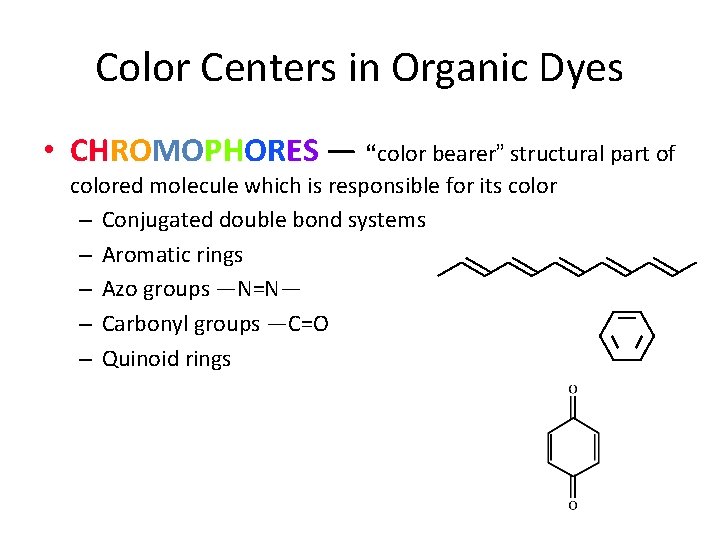
Color Centers in Organic Dyes • CHROMOPHORES — “color bearer” structural part of colored molecule which is responsible for its color – Conjugated double bond systems – Aromatic rings – Azo groups —N=N— – Carbonyl groups —C=O – Quinoid rings

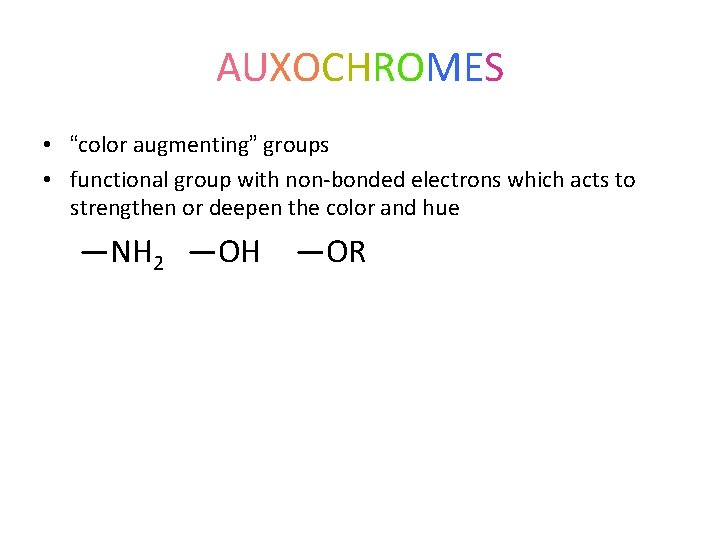
AUXOCHROMES • “color augmenting” groups • functional group with non-bonded electrons which acts to strengthen or deepen the color and hue —NH 2 —OH —OR

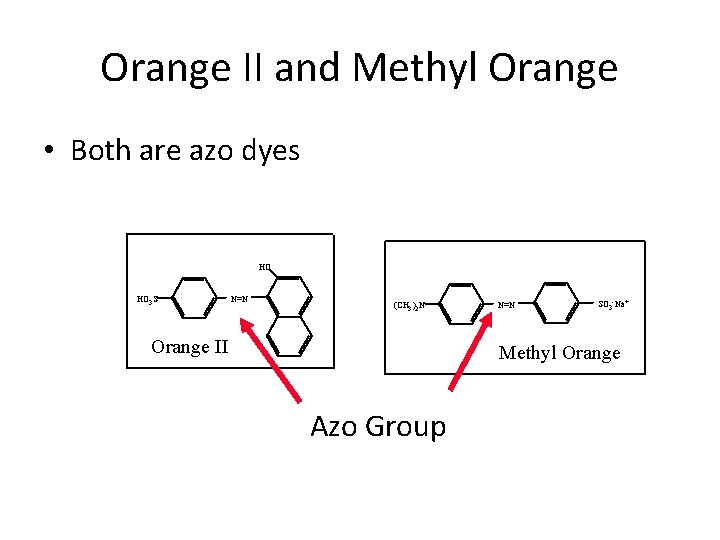
AZO Dyes • first prepared in 1863 Have widest range of colors of all dyes Contain the AZO Chromophore Generally lightfast R—N = N—R Azo group Brilliant colors ranging from reds to blues Methyl orange, Orange II, etc.

Orange II and Methyl Orange • Both are azo dyes HO HO 3 S N=N (CH 3 )2 N Orange II N=N SO 3 -Na+ Methyl Orange Azo Group

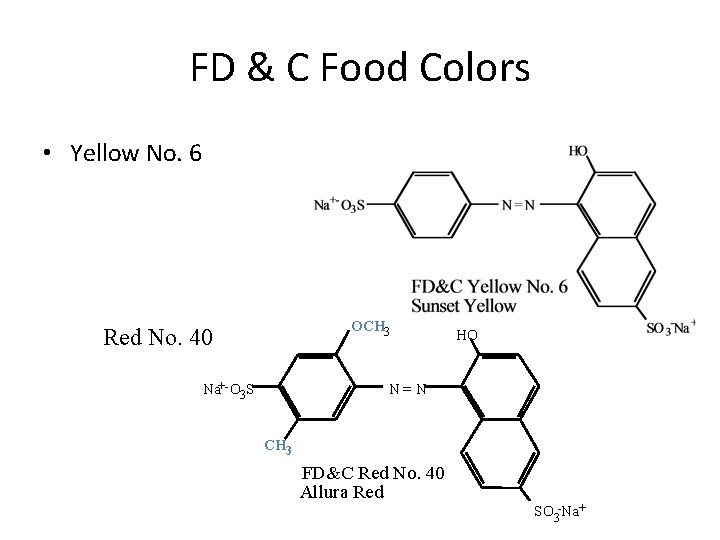
FD & C Food Colors • Yellow No. 6 OCH 3 Red No. 40 Na+- O 3 S HO N=N CH 3 FD&C Red No. 40 Allura Red SO 3 -Na+

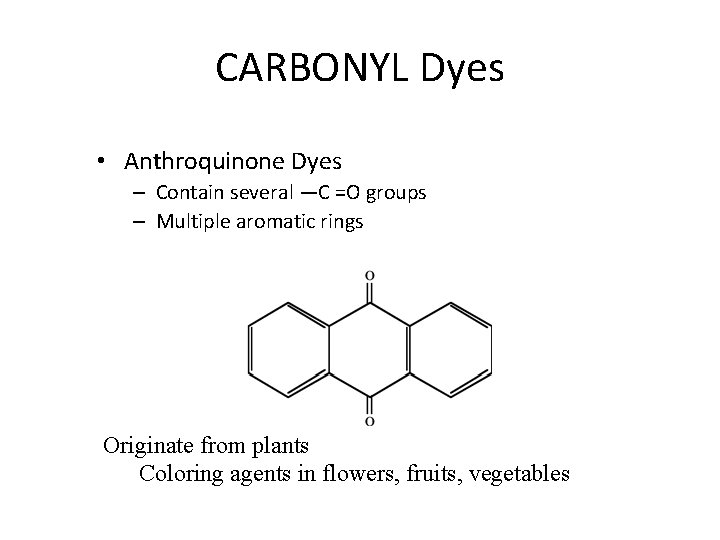
CARBONYL Dyes • Anthroquinone Dyes – Contain several —C =O groups – Multiple aromatic rings Originate from plants Coloring agents in flowers, fruits, vegetables

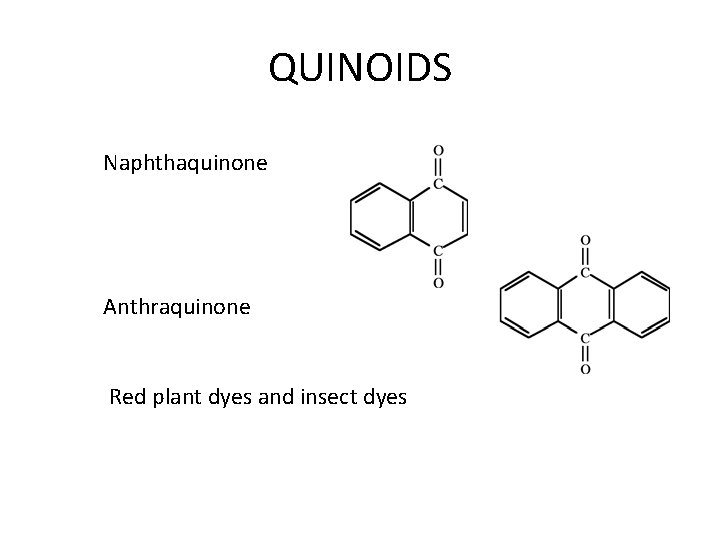
QUINOIDS Naphthaquinone Anthraquinone Red plant dyes and insect dyes

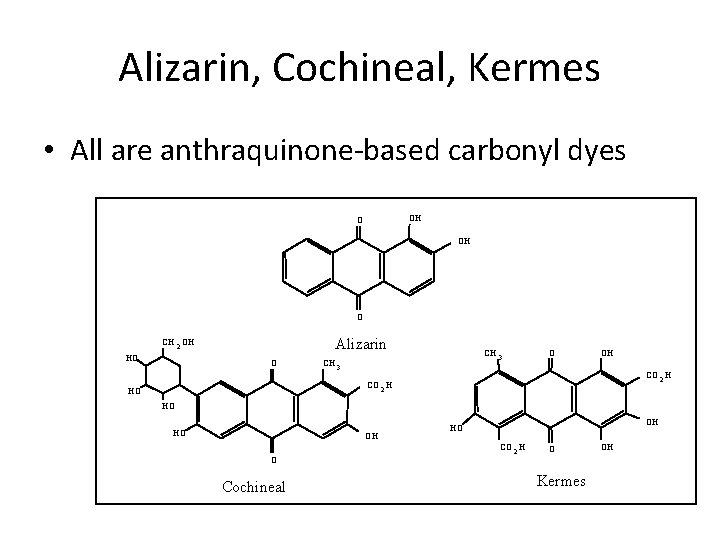
Alizarin, Cochineal, Kermes • All are anthraquinone-based carbonyl dyes OH O Alizarin CH 2 OH HO O CH 3 O OH CO 2 H HO HO HO OH O Cochineal OH HO CO 2 H O Kermes OH

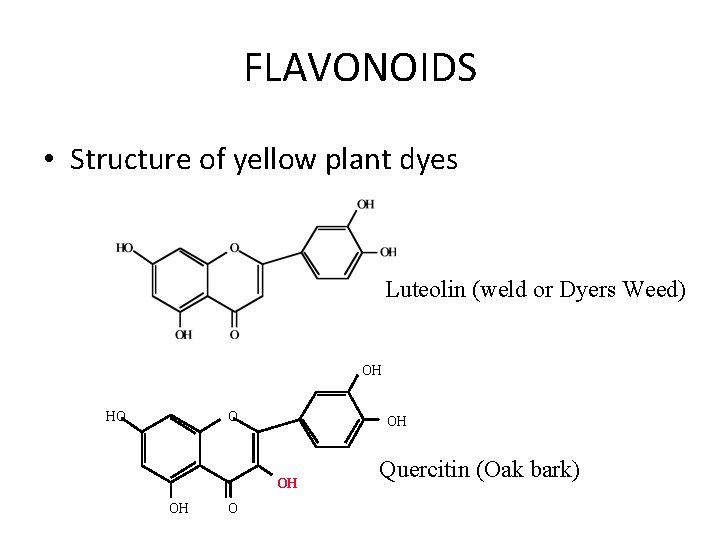
FLAVONOIDS • Structure of yellow plant dyes Luteolin (weld or Dyers Weed) OH HO O OH OH OH O Quercitin (Oak bark)

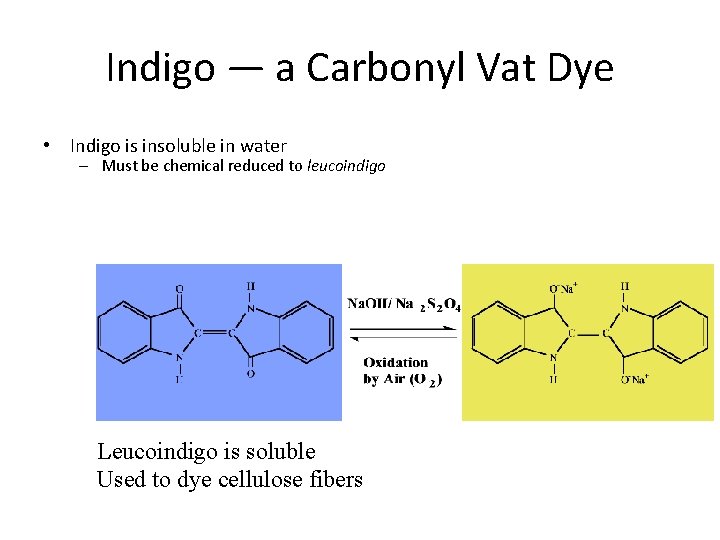
Indigo — a Carbonyl Vat Dye • Indigo is insoluble in water – Must be chemical reduced to leucoindigo Leucoindigo is soluble Used to dye cellulose fibers

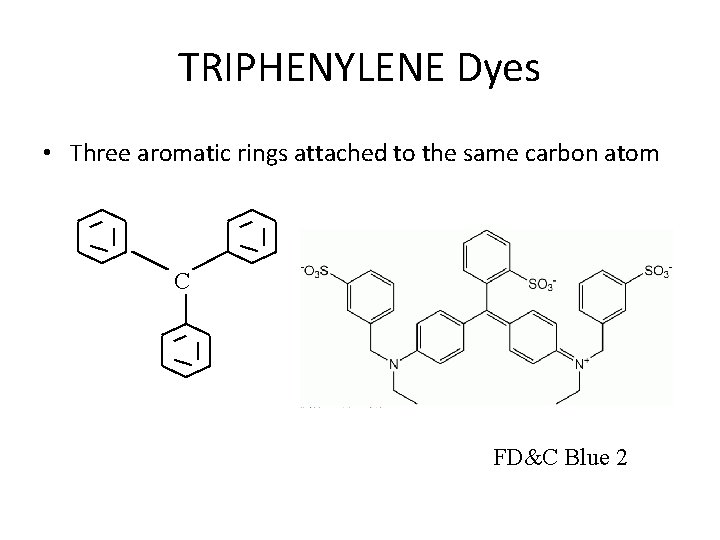
TRIPHENYLENE Dyes • Three aromatic rings attached to the same carbon atom C FD&C Blue 2

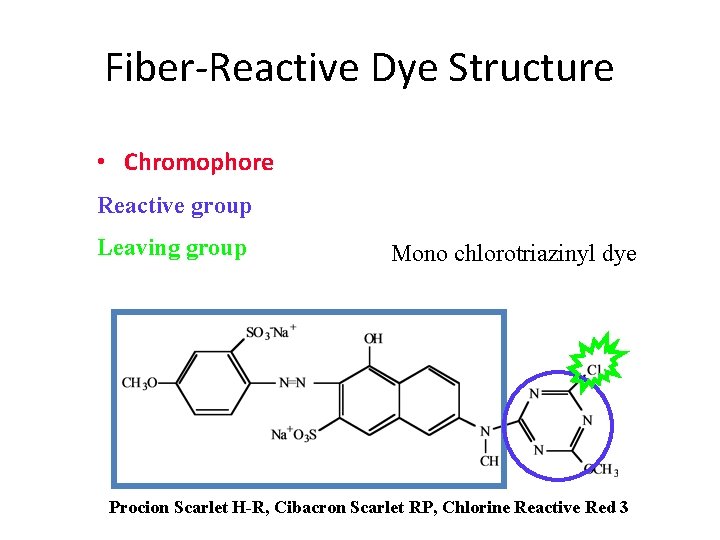
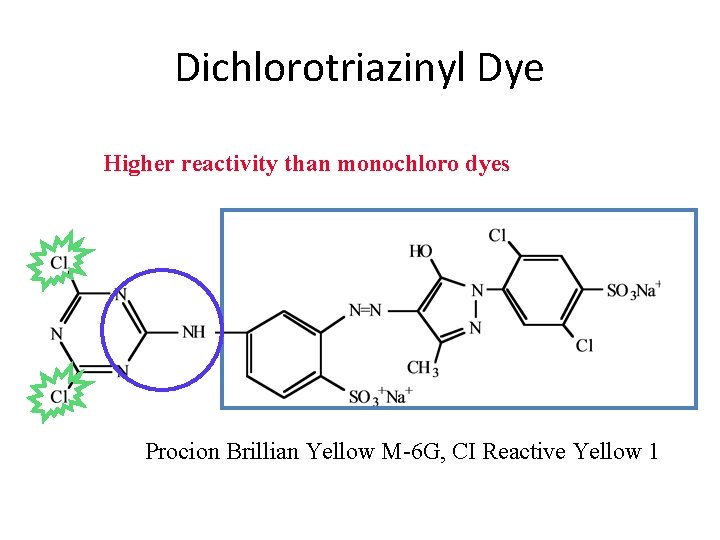
Fiber-Reactive Dye Structure • Chromophore Reactive group Leaving group Mono chlorotriazinyl dye Procion Scarlet H-R, Cibacron Scarlet RP, Chlorine Reactive Red 3

Dichlorotriazinyl Dye Higher reactivity than monochloro dyes Procion Brillian Yellow M-6 G, CI Reactive Yellow 1

Fastness in Dyes • Stability of dyes towards light • Dyes vary greatly in their lightfastness and colorfastness • Undergo photo-oxidation and photo-reduction by light — dyes fade and degrade

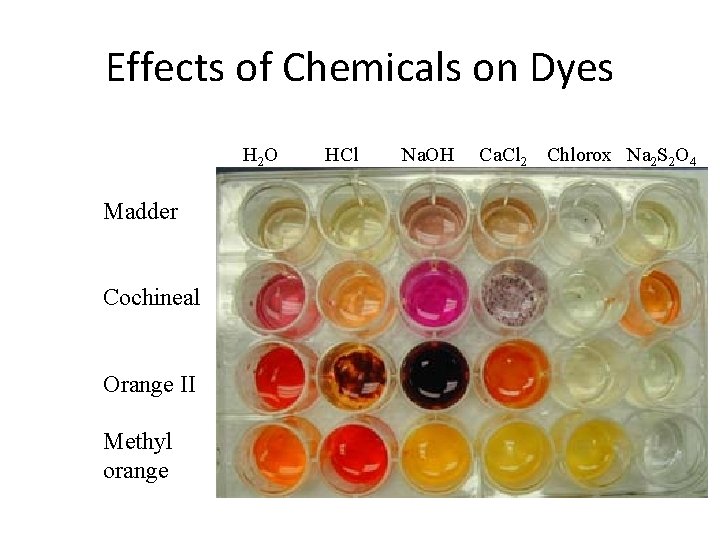
Effects of Chemicals on Dyes H 2 O Madder Cochineal Orange II Methyl orange HCl Na. OH Ca. Cl 2 Chlorox Na 2 S 2 O 4

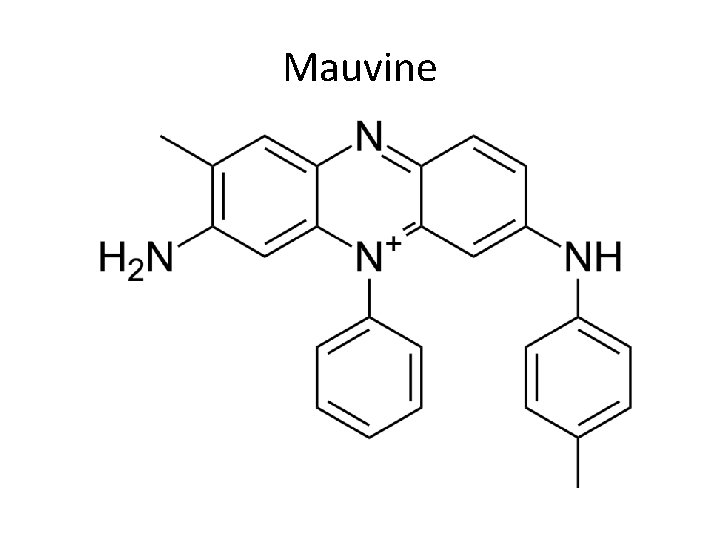
Mauvine
 Contoh serbuk gigi
Contoh serbuk gigi Coloranti naturali
Coloranti naturali L'expérience de l'amidon dans le pain
L'expérience de l'amidon dans le pain Hypericum adulteration red colorant
Hypericum adulteration red colorant Similar?
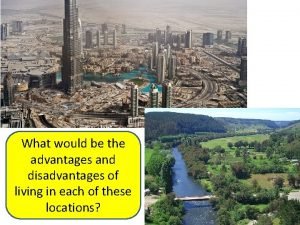
Similar? Disadvantages of clustered settlement
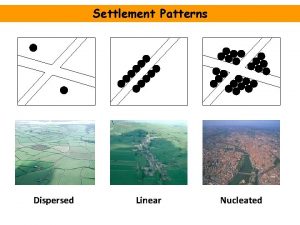
Disadvantages of clustered settlement Dispersed nucleated and linear settlements
Dispersed nucleated and linear settlements What is nucleated settlement
What is nucleated settlement Classification of suspension
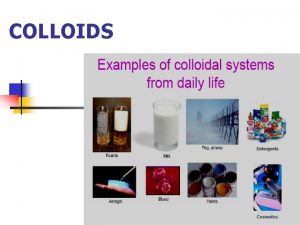
Classification of suspension Dispersion phase and dispersion medium
Dispersion phase and dispersion medium Dispersed system
Dispersed system Aix smit
Aix smit Ibm aix high availability
Ibm aix high availability Isoline definition ap human geography
Isoline definition ap human geography Seed dispersal by humans
Seed dispersal by humans Guava seed dispersal
Guava seed dispersal Dispersed
Dispersed Parallel sysplex licence charge
Parallel sysplex licence charge Dispersed rural settlement definition
Dispersed rural settlement definition The basic systems and services of a city
The basic systems and services of a city Dispersed phase
Dispersed phase Dispersed collective behavior
Dispersed collective behavior Red dye penetration test
Red dye penetration test Bianca dye
Bianca dye Azo dye chemical structure
Azo dye chemical structure Fawaz aldabbagh
Fawaz aldabbagh Odor of aniline
Odor of aniline Engine disassembly procedure
Engine disassembly procedure Allergy to hair dye icd 10
Allergy to hair dye icd 10 Mypa for bacillus cereus dye
Mypa for bacillus cereus dye Ultrasonic textile dye homogenizer
Ultrasonic textile dye homogenizer Prone
Prone Self sealability test for rubber closures
Self sealability test for rubber closures Cholangeogram
Cholangeogram Gallbladder and biliary system
Gallbladder and biliary system