Dye Colorant which is homogeneously dispersed in the






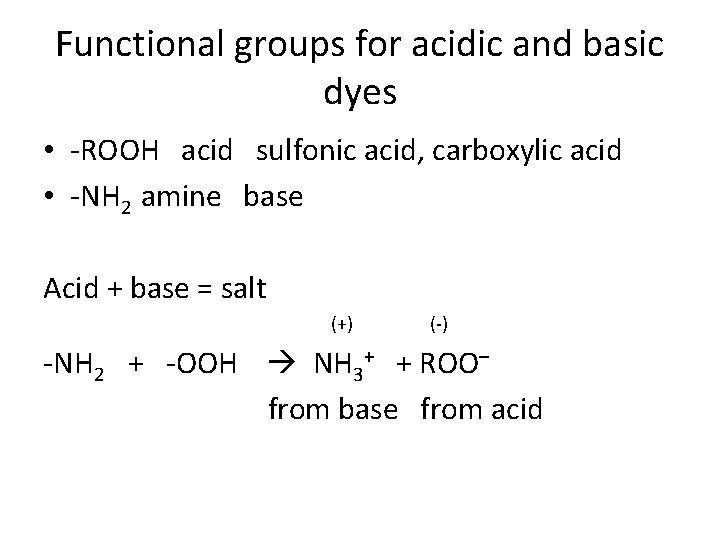


- Slides: 34

Dye: • Colorant which is homogeneously dispersed in the dye medium • Usually soluble • Naturally occurring or synthetic organics

Pazyryk carpet (Siberia) 5 th century BC Peruvian textile 600200 B. C.

Medieval Guilds of Master Dyers • Formed in Germany in the 12 th century • Apprentice system • Dye recipes were heavily guarded secrets

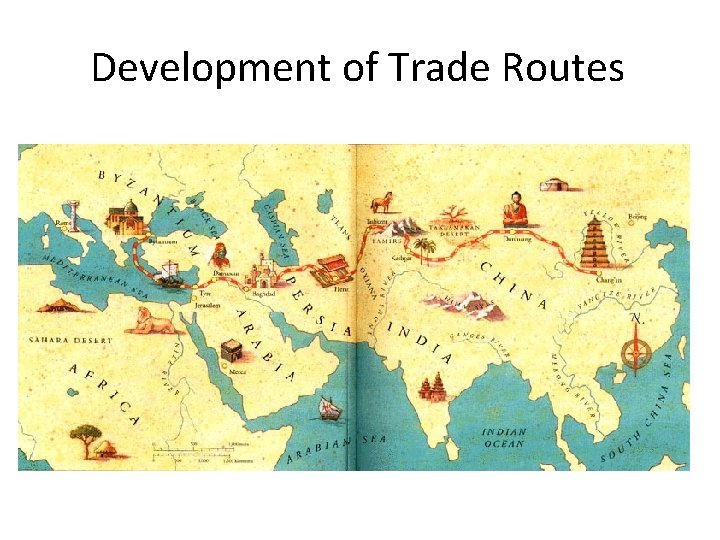
Development of Trade Routes • Textile trade China Europe – beginnings in 2, 000 B. C. – the “Silk Road” – sea routes from the Americas (15 th century) – East India Trading Company (17 th century) • Exchange of not only textiles but also designs, ideas and dyestuffs

Three Classes of Natural Dyes • Substantive dyes – Easiest to apply to fibers – No need to use other substances to enhance dyeing • Vat dyes – Involve chemical change of dye through “fermentation” or “chemical reduction” to make it soluble • Adjective dyes (Mordant dyes) – Require another chemical to develop color and “fix” it to the fiber

Dyes from Snails • Tyrian Purple or “Royal Blue” • 9000 snails to obtain 1 g of dye • Used primarily before 8 th century A. D. to dye wool and silk • Chemically it is 6, 6’dibromoindigo

Dyes from Bugs • Kermes — the most ancient dye in Europe 70, 000 female oak beetles produce 1 pound dye • Cochineal — Mexico and Central America Mexican cactus beetle

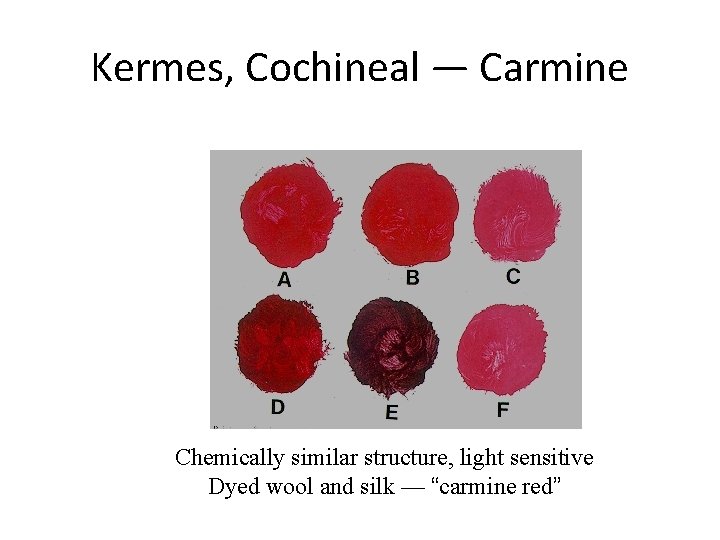
Kermes, Cochineal — Carmine Chemically similar structure, light sensitive Dyed wool and silk — “carmine red”

Dyes From Plants • Indigo — used since 2000 B. C. – – – Extracted from Indigofera tinctoria “Navy Blue” of English sailors Blue jeans Insoluble in water Must be chemical reduced to soluble leucoindigo to use as dye • Woad – Member of the mustard family – A common weed in temperate climates – Leaves contain same chemical as indigo but in lower amounts – Celtic war paint and tattoos Braveheart – Blue robes of priests

More Dyes From Plants • Madder — “Turkey Red” – Root of madder plant found in Europe and Asia – Prepared as a “lake” with Al(OH)3 – British “Redcoats” – Alexander the Great used it to simulate blood Chemically — Alizarin and Pupurin Synthetic alizarin prepared in 1875

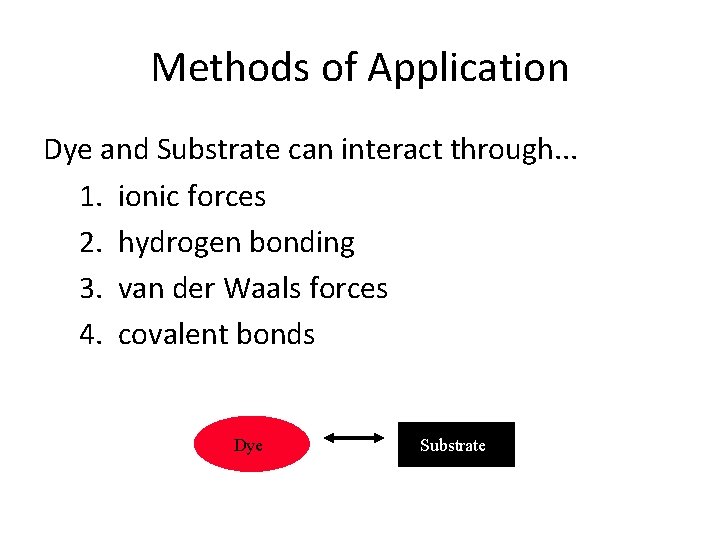
Methods of Application Dye and Substrate can interact through. . . 1. ionic forces 2. hydrogen bonding 3. van der Waals forces 4. covalent bonds Dye Substrate

Types of Dyes by Application • • • Acid Dyes Basic Dyes Mordant Dyes Direct Dyes Fiber-reactive Dyes Vat Dyes
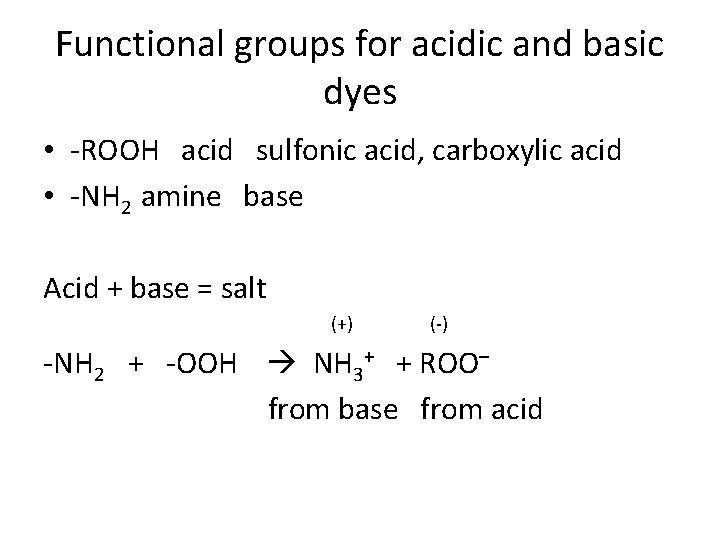
Functional groups for acidic and basic dyes • -ROOH acid sulfonic acid, carboxylic acid • -NH 2 amine base Acid + base = salt (+) (-) -NH 2 + -OOH NH 3+ + ROO– from base from acid

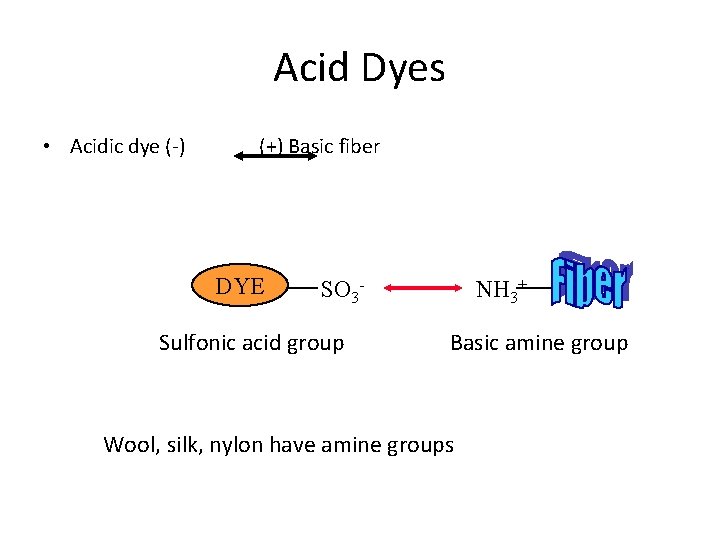
Acid Dyes • Acidic dye (-) (+) Basic fiber DYE NH 3+ SO 3 - Sulfonic acid group Basic amine group Wool, silk, nylon have amine groups

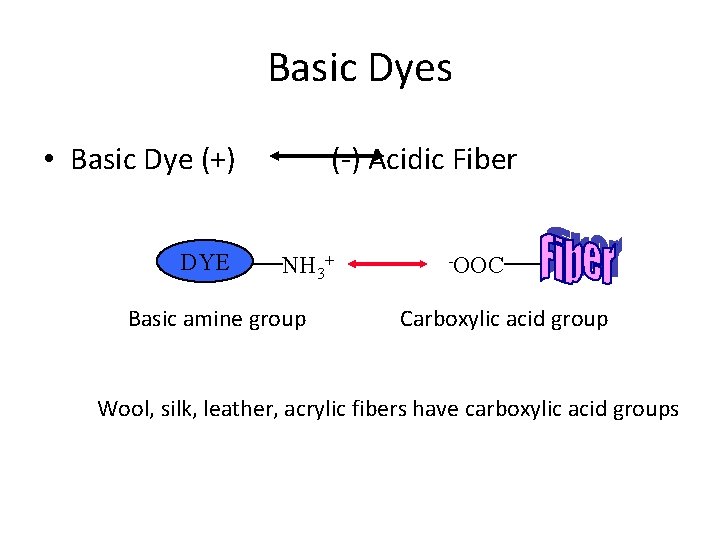
Basic Dyes • Basic Dye (+) DYE (-) Acidic Fiber NH 3+ Basic amine group -OOC Carboxylic acid group Wool, silk, leather, acrylic fibers have carboxylic acid groups

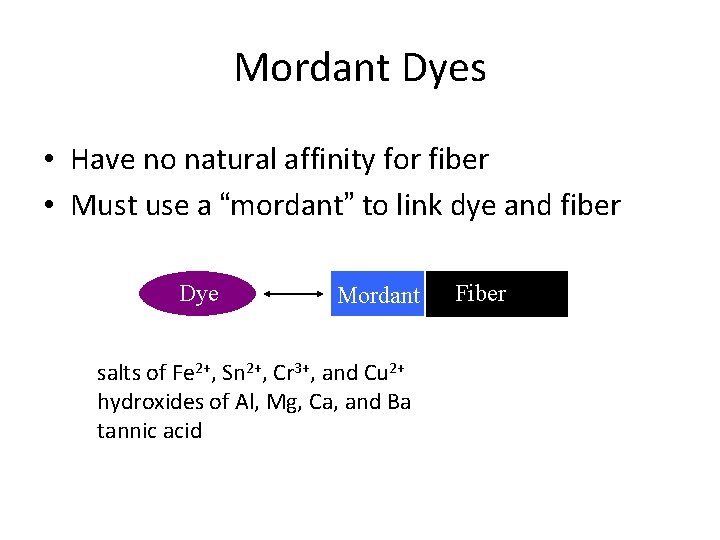
Mordant Dyes • Have no natural affinity for fiber • Must use a “mordant” to link dye and fiber Dye Mordant salts of Fe 2+, Sn 2+, Cr 3+, and Cu 2+ hydroxides of Al, Mg, Ca, and Ba tannic acid Fiber

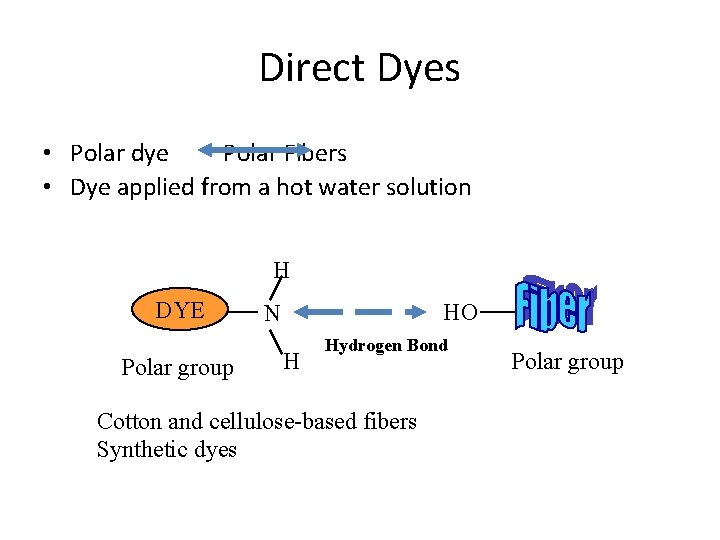
Direct Dyes • Polar dye Polar Fibers • Dye applied from a hot water solution H DYE Polar group HO N H Hydrogen Bond Cotton and cellulose-based fibers Synthetic dyes Polar group

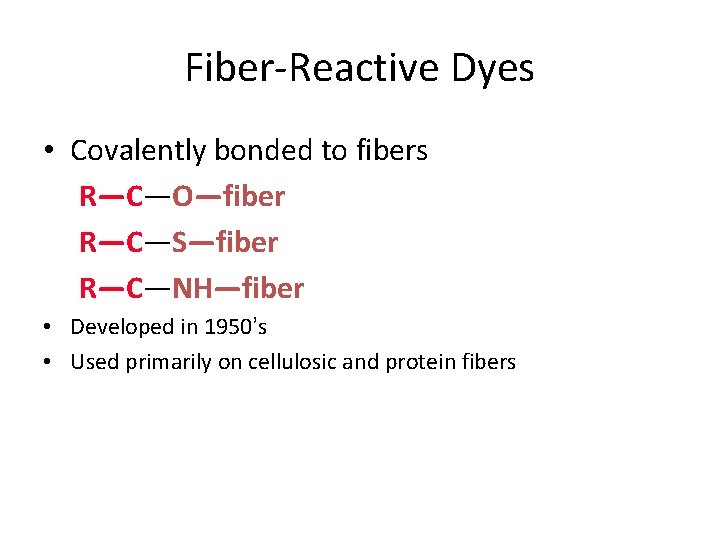
Fiber-Reactive Dyes • Covalently bonded to fibers R—C—O—fiber R—C—S—fiber R—C—NH—fiber • Developed in 1950’s • Used primarily on cellulosic and protein fibers

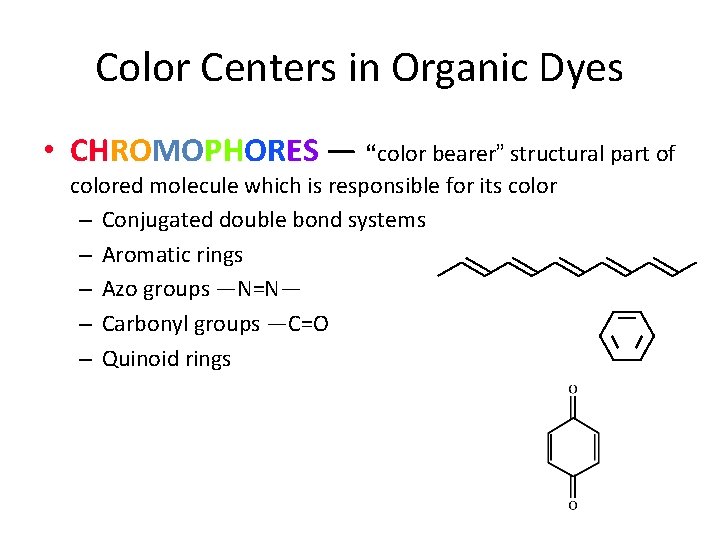
Color Centers in Organic Dyes • CHROMOPHORES — “color bearer” structural part of colored molecule which is responsible for its color – Conjugated double bond systems – Aromatic rings – Azo groups —N=N— – Carbonyl groups —C=O – Quinoid rings

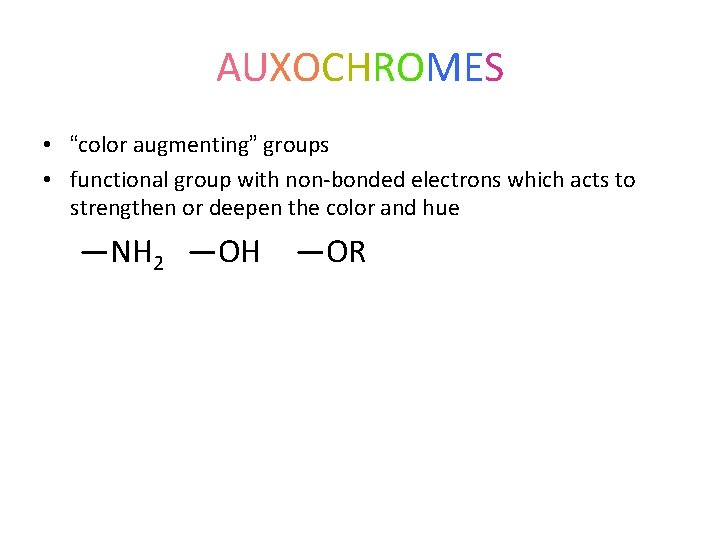
AUXOCHROMES • “color augmenting” groups • functional group with non-bonded electrons which acts to strengthen or deepen the color and hue —NH 2 —OH —OR

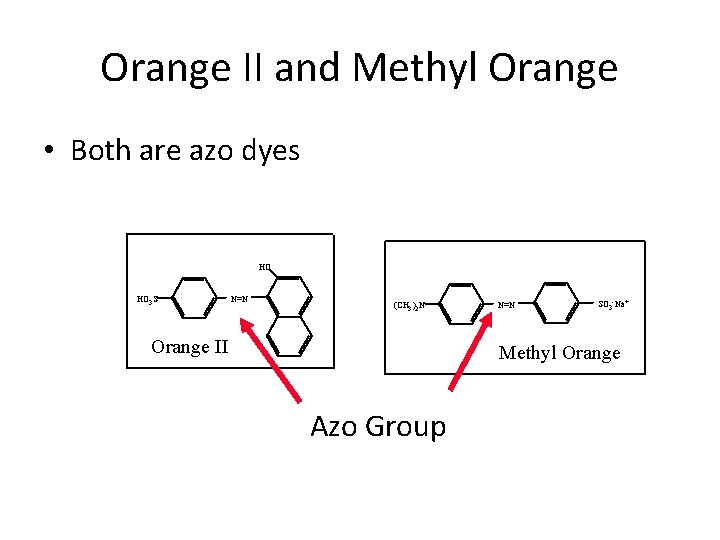
AZO Dyes • first prepared in 1863 Have widest range of colors of all dyes Contain the AZO Chromophore Generally lightfast R—N = N—R Azo group Brilliant colors ranging from reds to blues Methyl orange, Orange II, etc.

Orange II and Methyl Orange • Both are azo dyes HO HO 3 S N=N (CH 3 )2 N Orange II N=N SO 3 -Na+ Methyl Orange Azo Group

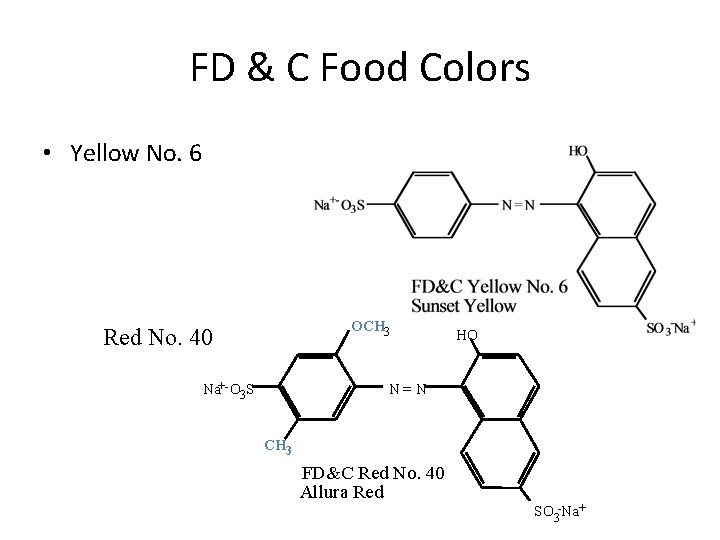
FD & C Food Colors • Yellow No. 6 OCH 3 Red No. 40 Na+- O 3 S HO N=N CH 3 FD&C Red No. 40 Allura Red SO 3 -Na+

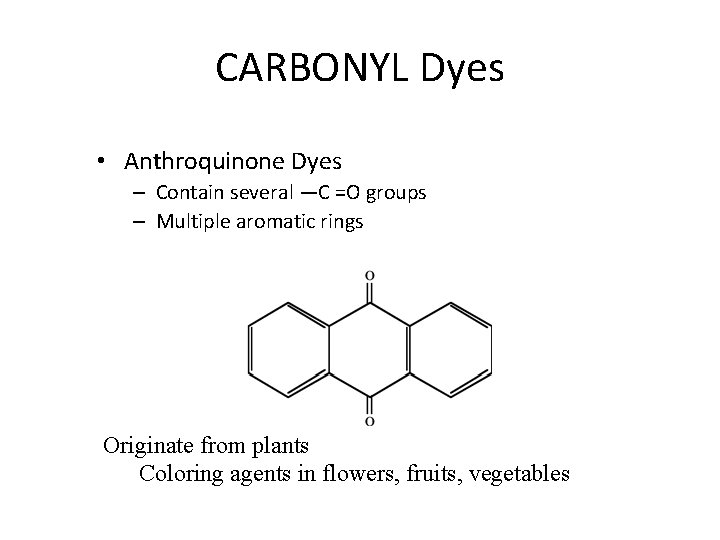
CARBONYL Dyes • Anthroquinone Dyes – Contain several —C =O groups – Multiple aromatic rings Originate from plants Coloring agents in flowers, fruits, vegetables

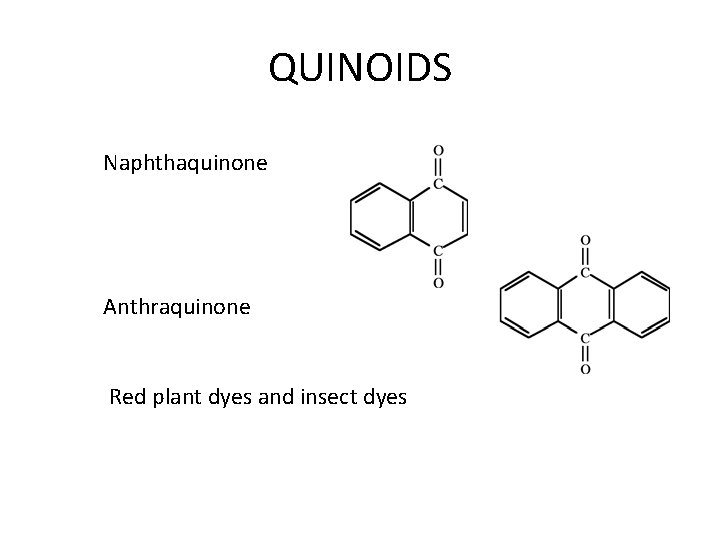
QUINOIDS Naphthaquinone Anthraquinone Red plant dyes and insect dyes

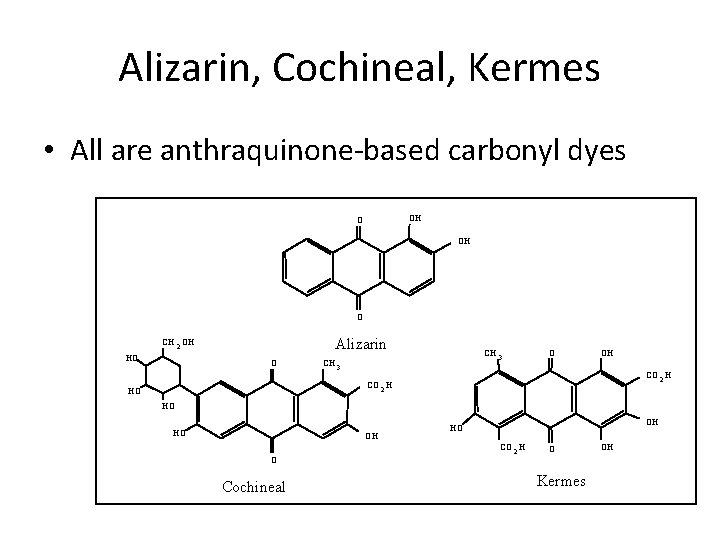
Alizarin, Cochineal, Kermes • All are anthraquinone-based carbonyl dyes OH O Alizarin CH 2 OH HO O CH 3 O OH CO 2 H HO HO HO OH O Cochineal OH HO CO 2 H O Kermes OH

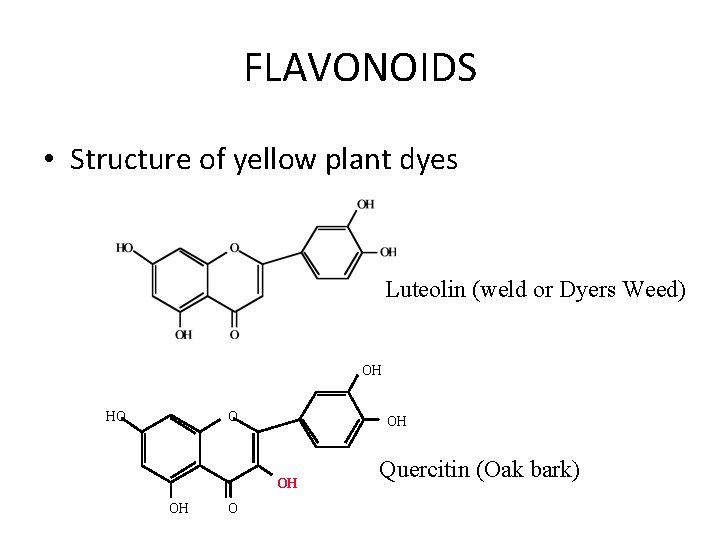
FLAVONOIDS • Structure of yellow plant dyes Luteolin (weld or Dyers Weed) OH HO O OH OH OH O Quercitin (Oak bark)

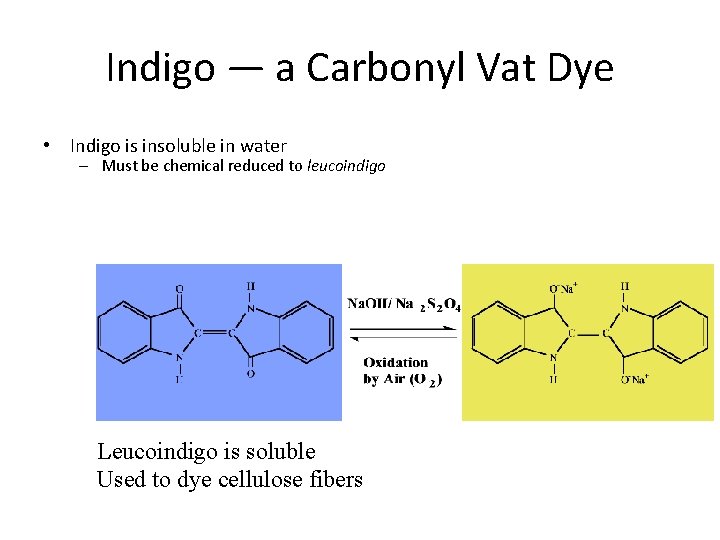
Indigo — a Carbonyl Vat Dye • Indigo is insoluble in water – Must be chemical reduced to leucoindigo Leucoindigo is soluble Used to dye cellulose fibers

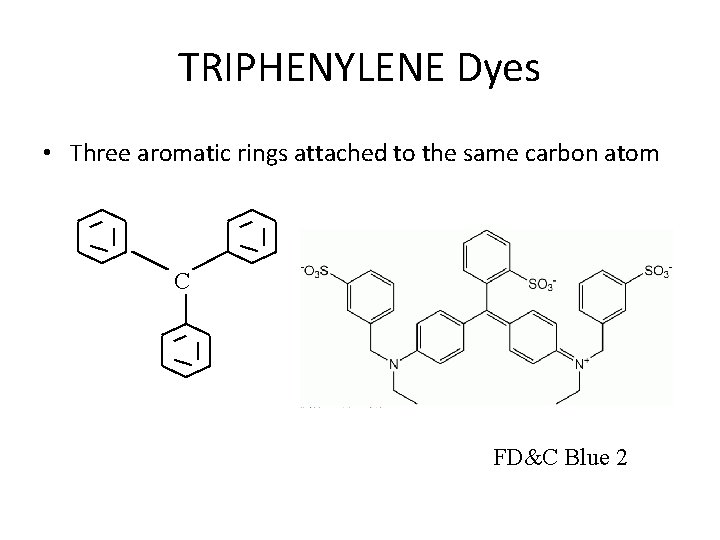
TRIPHENYLENE Dyes • Three aromatic rings attached to the same carbon atom C FD&C Blue 2

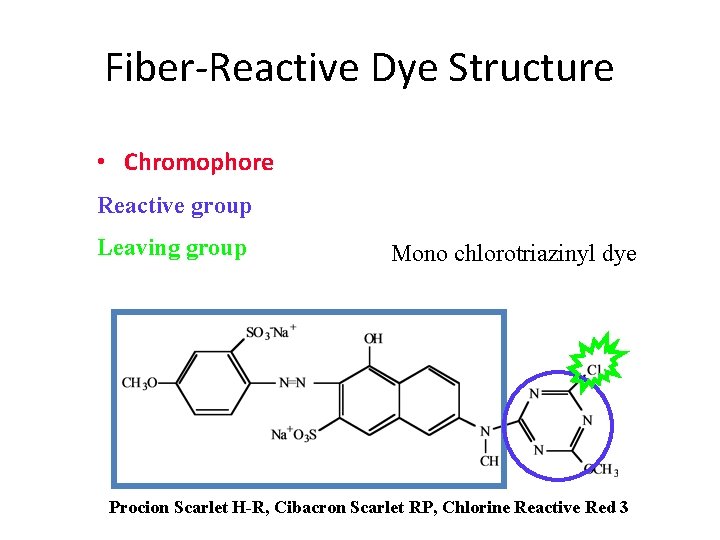
Fiber-Reactive Dye Structure • Chromophore Reactive group Leaving group Mono chlorotriazinyl dye Procion Scarlet H-R, Cibacron Scarlet RP, Chlorine Reactive Red 3

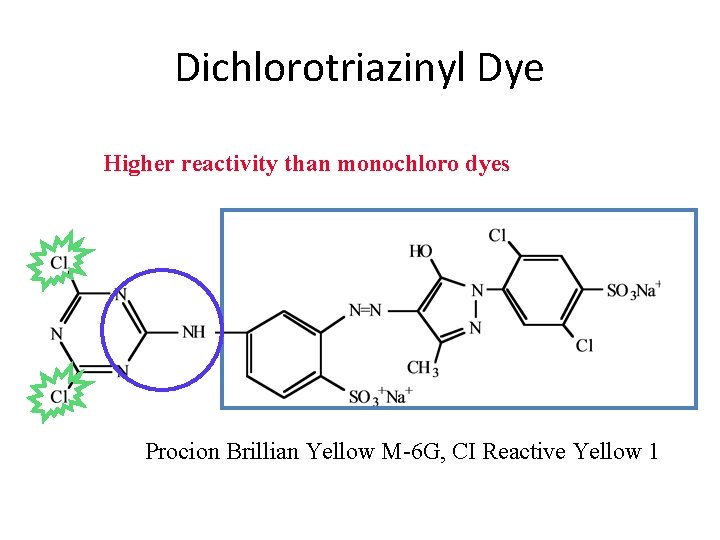
Dichlorotriazinyl Dye Higher reactivity than monochloro dyes Procion Brillian Yellow M-6 G, CI Reactive Yellow 1

Fastness in Dyes • Stability of dyes towards light • Dyes vary greatly in their lightfastness and colorfastness • Undergo photo-oxidation and photo-reduction by light — dyes fade and degrade

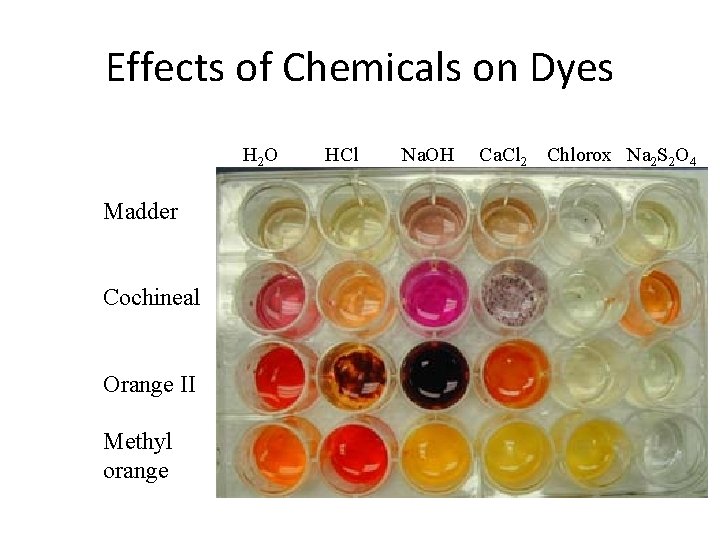
Effects of Chemicals on Dyes H 2 O Madder Cochineal Orange II Methyl orange HCl Na. OH Ca. Cl 2 Chlorox Na 2 S 2 O 4

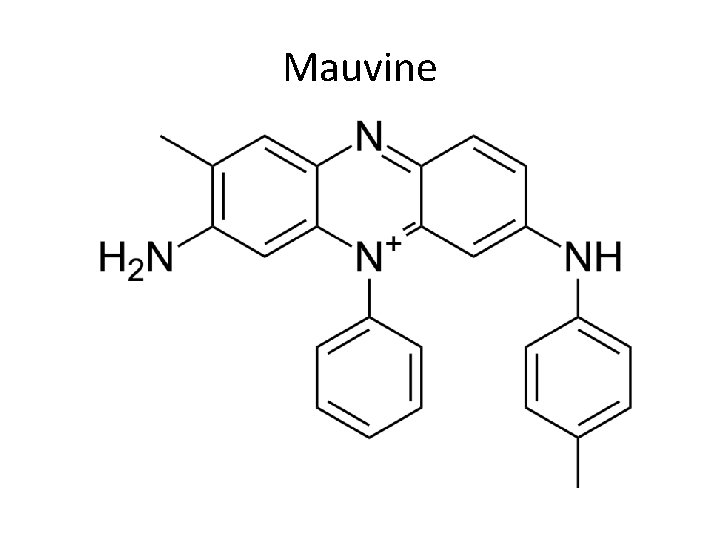
Mauvine