Dvidas denuccigdenucci com Arquivo Protocol development Evaluation and




![D [%] [ng/ml] [min] [h] Socra. Tec R & D D [%] [ng/ml] [min] [h] Socra. Tec R & D](https://slidetodoc.com/presentation_image/01b510cb1121540fc774e63938696128/image-6.jpg)
![D [%] [ng/ml] [min] [h] D [%] [ng/ml] [min] [h]](https://slidetodoc.com/presentation_image/01b510cb1121540fc774e63938696128/image-7.jpg)























































- Slides: 81

Dúvidas denucci@gdenucci. com Arquivo Protocol development Evaluation and Decision. ppt Site www. gdenucci. com


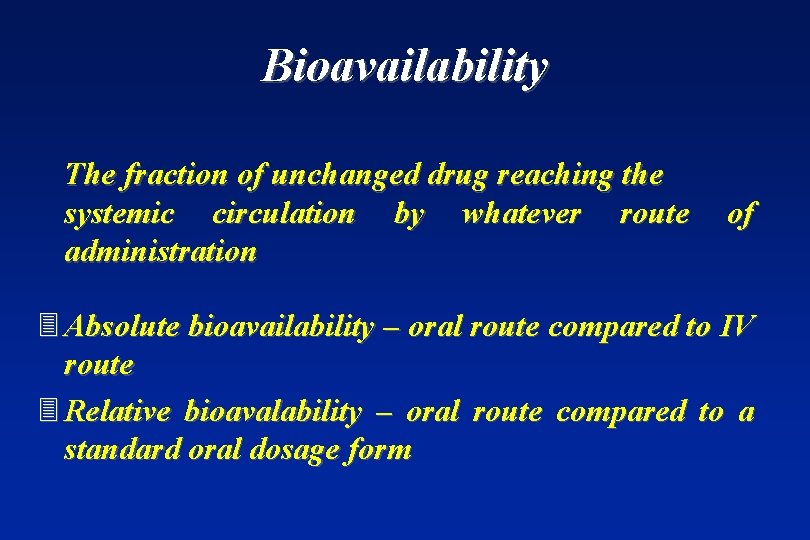
Bioavailability The fraction of unchanged drug reaching the systemic circulation by whatever route administration of 3 Absolute bioavailability – oral route compared to IV route 3 Relative bioavalability – oral route compared to a standard oral dosage form

BIOAVAILABILITY CAN BE DETERMINED BY MEASUREMENTS OF 3 Concentration of the active drug or metabolite(s) in biological fluid as a function of time. 3 Urinary excretion of the active drug or metabolite(s) as a function of time. 3 Any appropriate acute pharmacological effect.

Equivalence 3 Comparative bioavailability (bioequivalence) studies in humans 3 Comparative pharmacodynamic studies in humans 3 Comparative clinical trials 3 In vitro dissolution tests (in certain circumstances – approximately 25%)
![D ngml min h Socra Tec R D D [%] [ng/ml] [min] [h] Socra. Tec R & D](https://slidetodoc.com/presentation_image/01b510cb1121540fc774e63938696128/image-6.jpg)
D [%] [ng/ml] [min] [h] Socra. Tec R & D
![D ngml min h D [%] [ng/ml] [min] [h]](https://slidetodoc.com/presentation_image/01b510cb1121540fc774e63938696128/image-7.jpg)
D [%] [ng/ml] [min] [h]

Fundamental Bioequivalence Assumption When two drug products are equivalent in the rate and extent to which the active drug ingredient or therapeutic moiety is absorbed and becomes available at the site of drug action, it is assumed that they will be therapeutically equivalent.

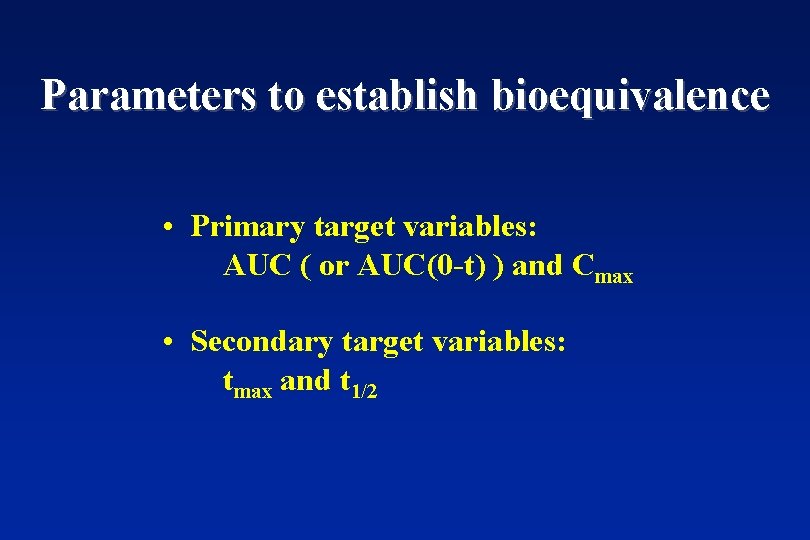
Parameters to establish bioequivalence • Primary target variables: AUC ( or AUC(0 -t) ) and Cmax • Secondary target variables: tmax and t 1/2

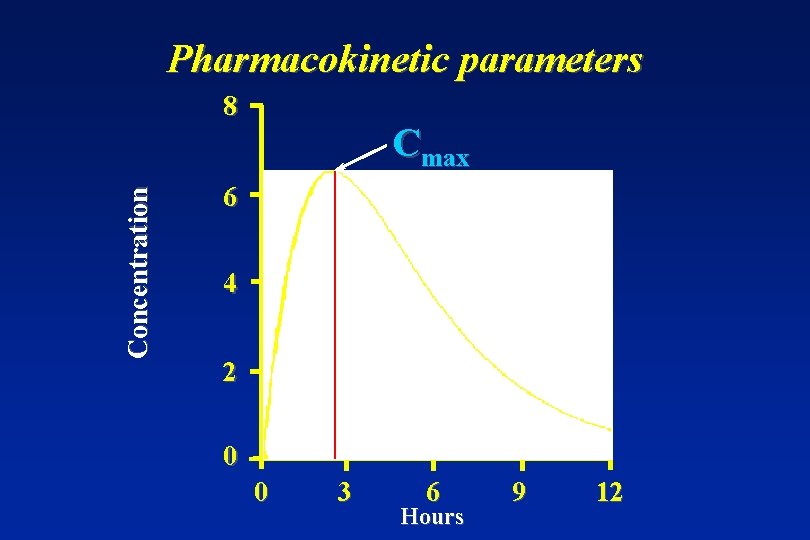
Pharmacokinetic parameters Concentration 8 Cmax 6 4 2 tmax 0 0 3 6 Hours 9 12

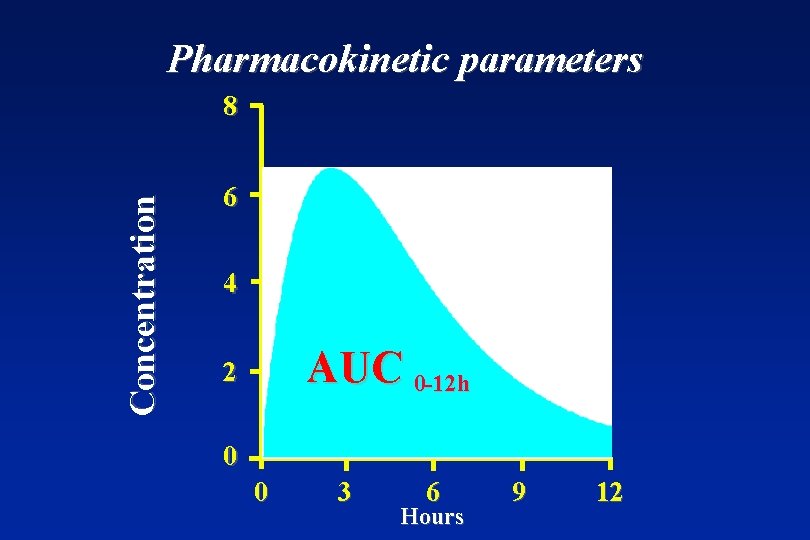
Pharmacokinetic parameters Concentration 8 6 4 AUC 0 -12 h 2 0 0 3 6 Hours 9 12

Design and analysis of average bioequivalence studies

Design • 2 x 2 cross-over Sequence Period 1 Period 2 1 Reference Test 2 Test Reference • washout to avoid carry-over effects – also in food-interaction – drug-interaction studies may consider “one-sequence cross-over” or parallel group designs

Bioequivalence study using two parallel groups + study can be completed quickly, no washout necessary - analysis based on inter-subject variability, which is generally much higher than intra-subject variability: much more subjects needed

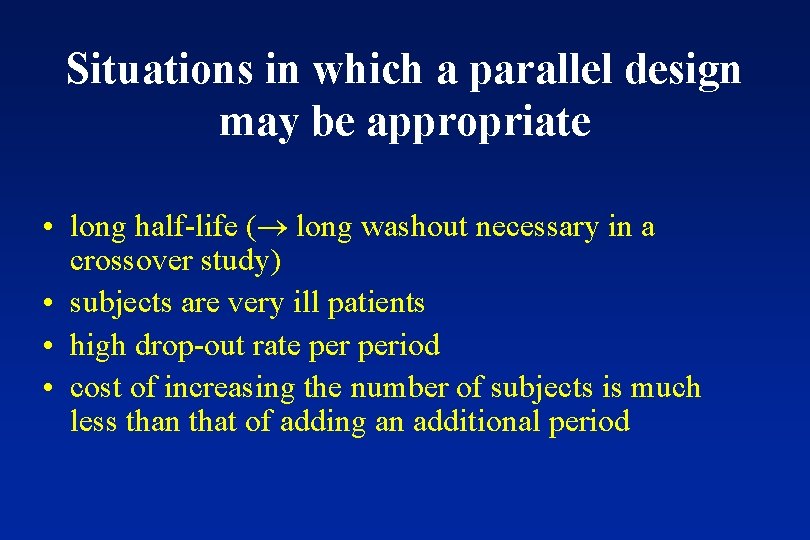
Situations in which a parallel design may be appropriate • long half-life ( long washout necessary in a crossover study) • subjects are very ill patients • high drop-out rate period • cost of increasing the number of subjects is much less than that of adding an additional period

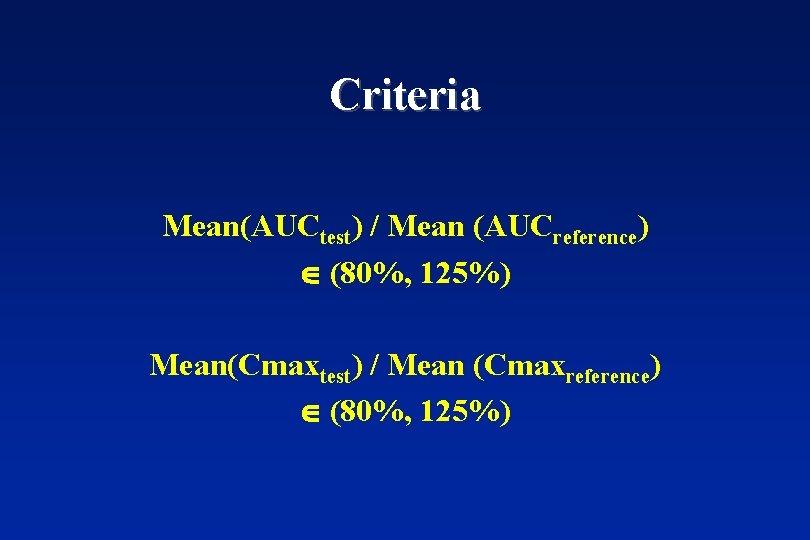
Criteria Mean(AUCtest) / Mean (AUCreference) (80%, 125%) Mean(Cmaxtest) / Mean (Cmaxreference) (80%, 125%)

Socra. Tec R & D

Socra. Tec R & D

Socra. Tec R & D



Problemas de Bioequivalência 1 - Produto de referência 2 - Critérios para aferição da bioequivalência (intervalos muito grandes ou muito curtos dependendo do produto) 3 - Validade do teste de bioequivalência 4 - Desenho dos estudos (dose única, dose múltipla, etc).

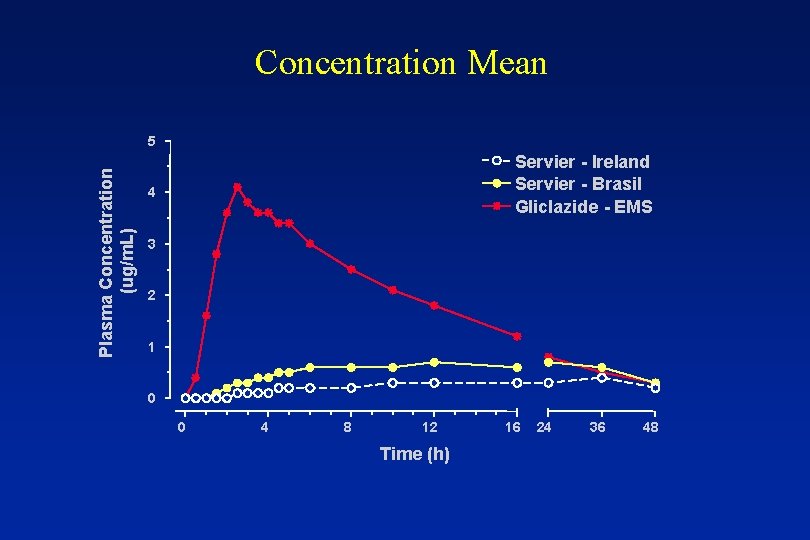
Concentration Mean

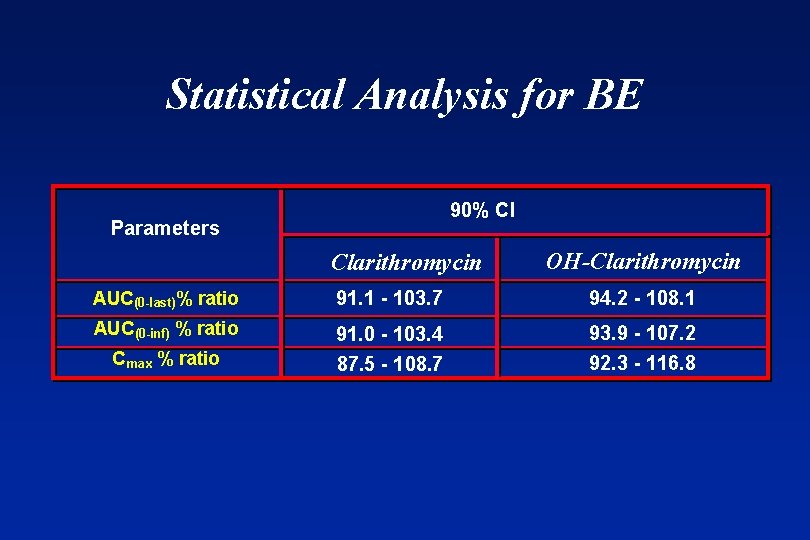
Statistical Analysis for BE 90% CI Parameters Clarithromycin OH Clarithromycin AUC(0 -last)% ratio 91. 1 - 103. 7 94. 2 - 108. 1 AUC(0 -inf) % ratio 91. 0 - 103. 4 Cmax % ratio 87. 5 - 108. 7 93. 9 - 107. 2 92. 3 - 116. 8

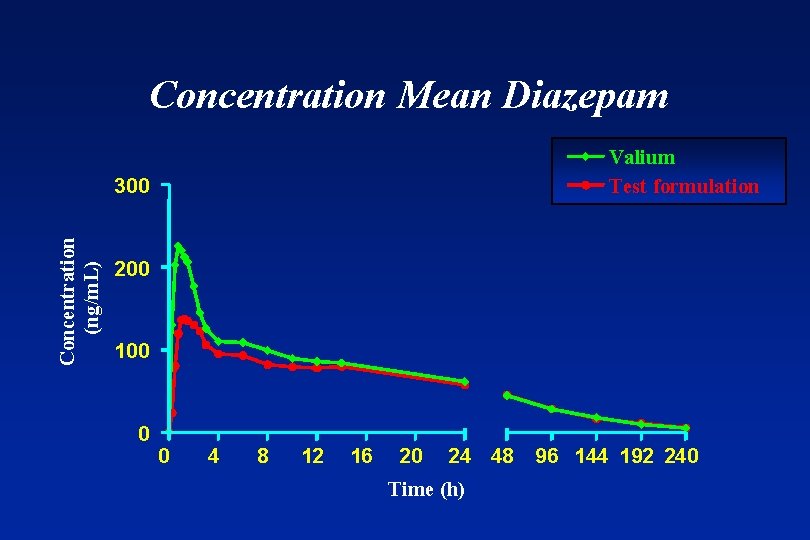
Concentration Mean Diazepam Valium Test formulation Concentration (ng/m. L) 300 200 100 0 0 4 8 12 16 20 24 48 Time (h) 96 144 192 240

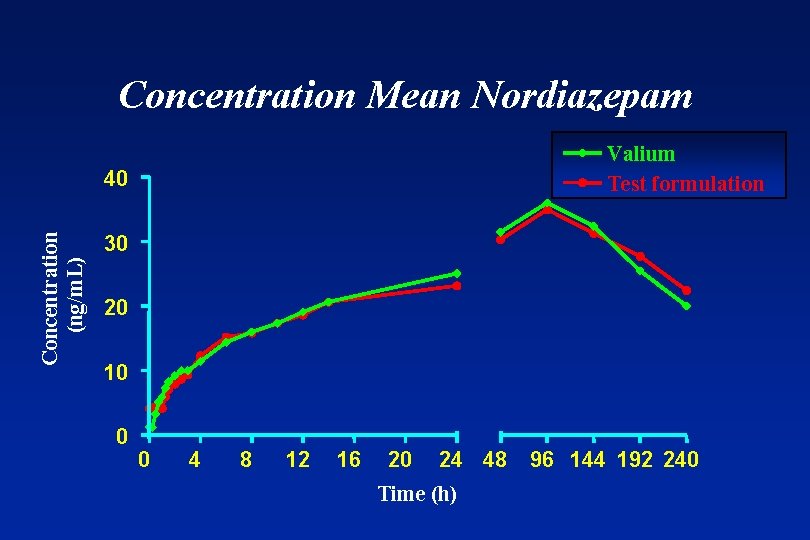
Concentration Mean Nordiazepam Valium Test formulation Concentration (ng/m. L) 40 30 20 10 0 0 4 8 12 16 20 24 48 Time (h) 96 144 192 240

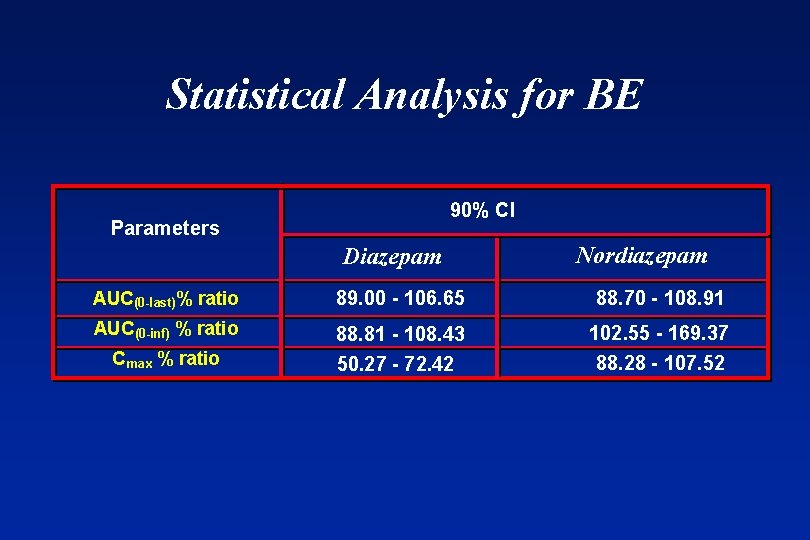
Statistical Analysis for BE 90% CI Parameters Diazepam Nordiazepam AUC(0 -last)% ratio 89. 00 - 106. 65 88. 70 - 108. 91 AUC(0 -inf) % ratio 88. 81 - 108. 43 Cmax % ratio 50. 27 - 72. 42 102. 55 - 169. 37 88. 28 - 107. 52

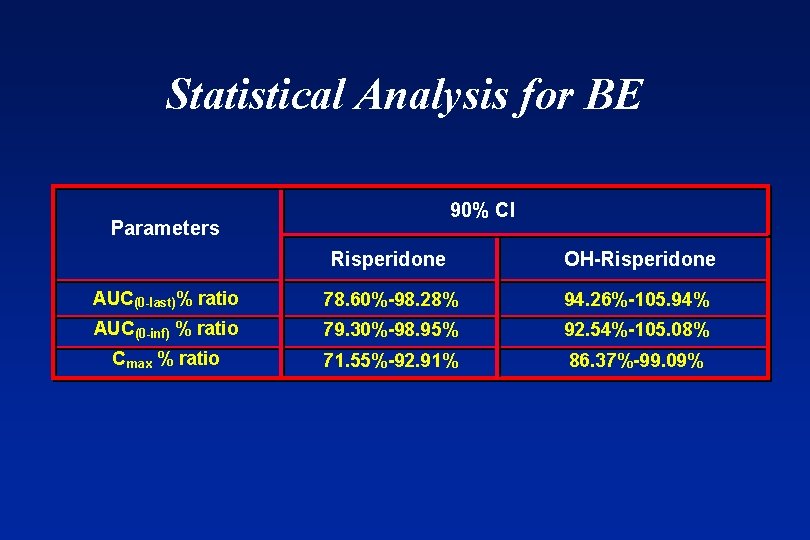
Statistical Analysis for BE 90% CI Parameters Risperidone OH-Risperidone AUC(0 -last)% ratio 78. 60%-98. 28% 94. 26%-105. 94% AUC(0 -inf) % ratio 79. 30%-98. 95% 92. 54%-105. 08% Cmax % ratio 71. 55%-92. 91% 86. 37%-99. 09%

How do get them? Ø Commercialy available Ø Chemical synthesis Ø Biological synthesis Ø Recovery from biological samples

Chemical synthesis Ø Expensive Ø Technically difficult

Biological synthesis Ø Enzymes (bacteria, microsomes, hepatocytes, etc). Ø Requires very good efficacy

Recovery from biological samples Ø Previous administration (recovery from urine) to volunteer

Not available Measurable (expressed as equivalents of parent)

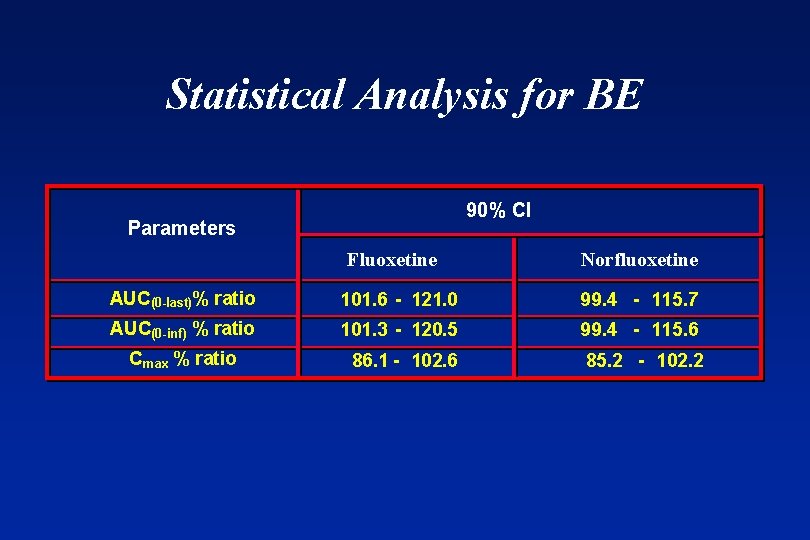
Statistical Analysis for BE 90% CI Parameters Fluoxetine Norfluoxetine AUC(0 -last)% ratio 101. 6 - 121. 0 99. 4 - 115. 7 AUC(0 -inf) % ratio 101. 3 - 120. 5 99. 4 - 115. 6 86. 1 - 102. 6 85. 2 - 102. 2 Cmax % ratio


Bioavailability study comparing one test formulation of Paracetamol / Dimethinden Maleate / Phenylephrine hydrochloride (Trimedal - tablet for 500 / 10 mg, respectively; NOVARTIS Biociências S. A. ) versus reference tablet products (Paracetamol 500 mg, Dimethinden Maleate 1. 0 mg, Phenylephrine hydrochloride 10 mg individually administered; NOVARTIS Biociências S. A. ) in healthy volunteers of both genders

OBJECTIVE • The present study was conducted to evaluate the pharmacokinetic profiles of the combination formulation – Trimedal [Paracetamol / Dimethindene / Phenylephrine] (formulation 1 – test product) against the individual administration of each of its compounds in order to evaluate the influence of the coadministration.

PLASMA CONCENTRATIONS VS. SCHEDULED BLOOD SAMPLING TIME USING IN LINEAR SCALE (PHENYLEPHRINE)

PLASMA CONCENTRATIONS VS. SCHEDULED BLOOD SAMPLING TIME USING IN LINEAR SCALE (DIMETHINDENE)

PLASMA CONCENTRATIONS VS. SCHEDULED BLOOD SAMPLING TIME USING IN LINEAR SCALE (PARACETAMOL)

DIMETHINDENE PHARMACOKINETIC PARAMETERS

PHENYLEPHRINE PHARMACOKINETIC PARAMETERS

PARACETAMOL PHARMACOKINETIC PARAMETERS

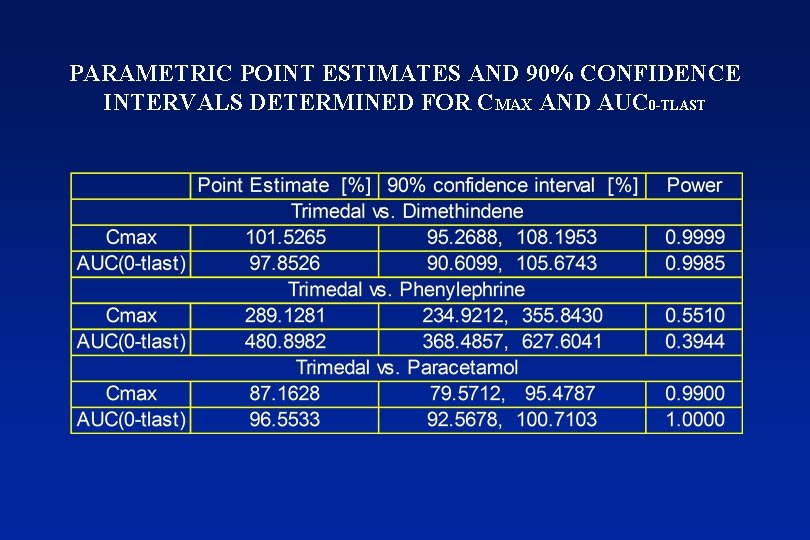
PARAMETRIC POINT ESTIMATES AND 90% CONFIDENCE INTERVALS DETERMINED FOR CMAX AND AUC 0 -TLAST

CONCLUSIONS From the above results it can be concluded: • DIMETHINDENE: the addition of Paracetamol and Phenylephrine in the Test Product does not interfere with the action of Dimethindene, since the Cmax and AUC(0 -last) pharmacokinetic parameters are within the expected values reported in the literature. • PHENYLEPHRINE: The results for Phenylephrine conform to the reported literature as a highly variable drug (power of 0. 39 for AUC in contrast with the power ~1. 00 obtained for the other formulation compounds). From the results it can be concluded that the addition of Paracetamol and Dimethindene in the combination formulation Trimedal (formulation 1 -test product) does not inhibit Phenylephrine absorption. Therefore it should not impair Phenylephrine therapeutic effect. • PARACETAMOL: it can be concluded that the addition of Dimethindene and Phenylephrine in the Test Product does not interfere with the action of Paracetamol, since the Cmax and AUC(0 -last) pharmacokinetic parameters are within the expected values reported in the literature.

Bioavailability Study comparing one Spironolactone formulation from Laboratórios Biosintética Ltda (tablet 100 mg) with a marketed reference product Aldactone (tablet 100 mg) in healthy volunteers

OBJECTIVE • The objective of this study was to the assessment of relative bioavailability of 2 products in order to determine whether they are bioequivalent. • Test: Aldactone - 100 mg tablet - single dose, oral administration; • Reference: Aldactone 100 mg tablet - single dose, oral administration;

PLASMA CONCENTRATIONS VS. SCHEDULED BLOOD SAMPLING TIME USING IN LINEAR SCALE (SPIRONOLACTONE)

PLASMA CONCENTRATIONS VS. SCHEDULED BLOOD SAMPLING TIME USING IN LINEAR SCALE (CANRENONE)

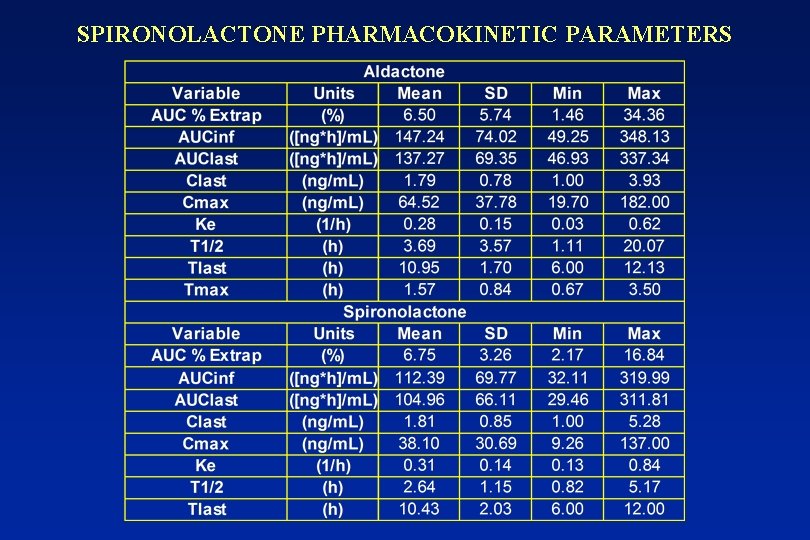
SPIRONOLACTONE PHARMACOKINETIC PARAMETERS

CANRENONE PHARMACOKINETIC PARAMETERS

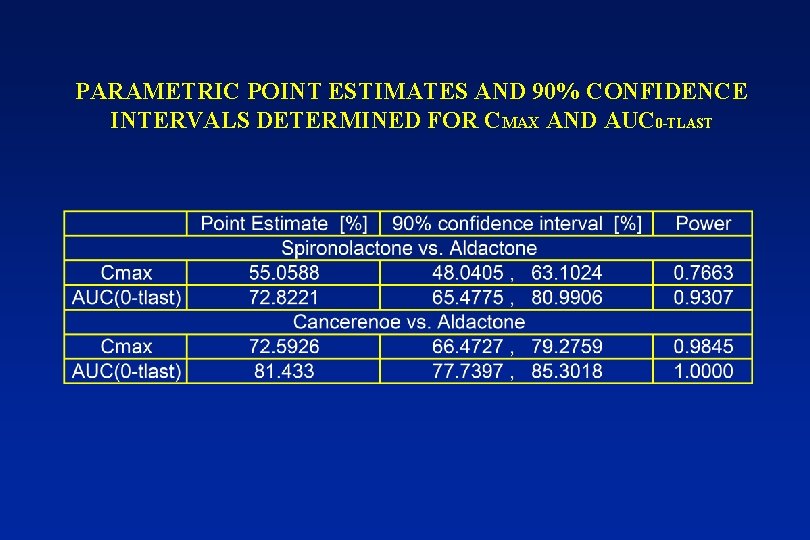
PARAMETRIC POINT ESTIMATES AND 90% CONFIDENCE INTERVALS DETERMINED FOR CMAX AND AUC 0 -TLAST

Bioequivalence Study comparing one Theophylline (capsule 300 mg) formulation from Novartis Biociências S. A. with a marketed reference product Teolong (capsule 300 mg) from Knoll Produtos Químicos Farmacêuticos Ltda. in healthy volunteers of both genders under fasting and fed conditions

OBJECTIVE • The objective of this study was to the assessment of relative bioavailability of 2 products in order to determine whether they are bioequivalent under fasting and fed conditions. • Test: Talofilina [Theophylline] - 300 mg - capsule single dose, oral administration; Novartis Biociências S. A. • Reference: Teolong [Theophylline] - 300 mg – capsule single dose, oral administration; Knoll

PLASMA CONCENTRATIONS VS. SCHEDULED BLOOD SAMPLING TIME USING IN LINEAR SCALE (THEOPHYLLINE)

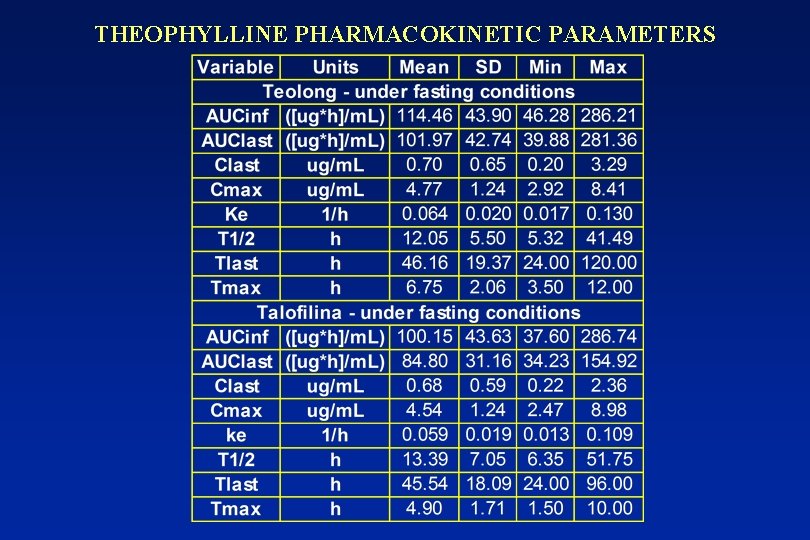
THEOPHYLLINE PHARMACOKINETIC PARAMETERS

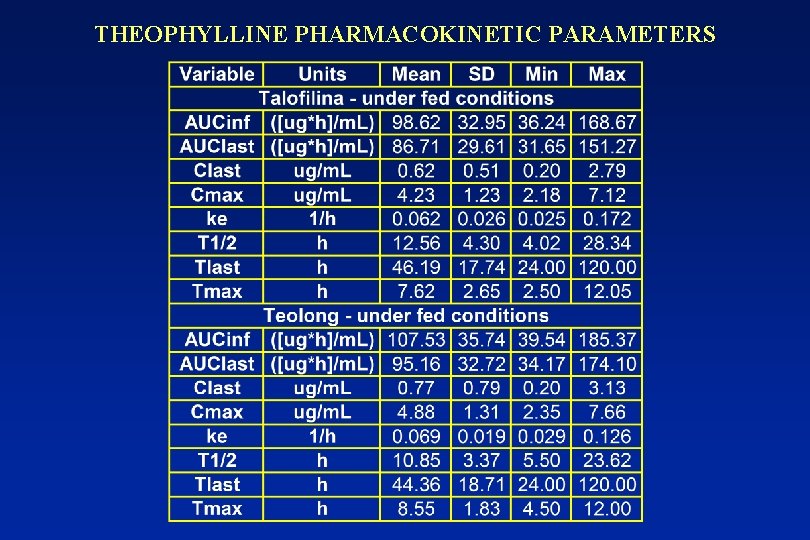
THEOPHYLLINE PHARMACOKINETIC PARAMETERS

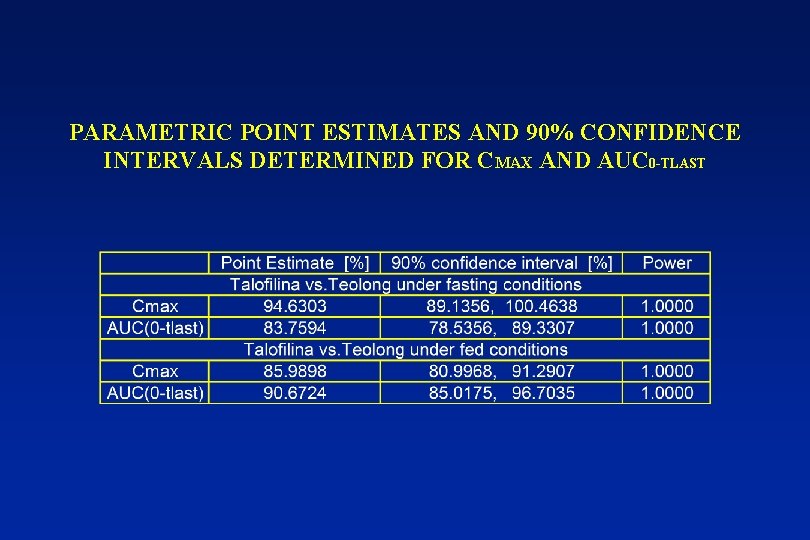
PARAMETRIC POINT ESTIMATES AND 90% CONFIDENCE INTERVALS DETERMINED FOR CMAX AND AUC 0 -TLAST























Concentration Mean Plasma Concentration (ug/m. L) 5 Servier - Ireland Servier - Brasil Gliclazide - EMS 4 3 2 1 0 0 4 8 12 Time (h) 16 24 36 48