Dutasteride Indian Experience Dr Sunil Shroff Professor HOD















- Slides: 52

Dutasteride – Indian Experience Dr. Sunil Shroff Professor & HOD, Dept. of Urology & Renal Transplantation Sri Ramachandra Medical College & Research Institution, Chennai E-mail: sshroff@vsnl. com or srmc 69@eth. net www. medindia. net / urology Dr. Sunil Shroff - Dutasteride 2004

BPH – Changing Patterns in Management • Over the last 10 years Treatment of BPH has changed dramatically • The emphasis is more towards symptom improvement and prevention of Clinical progression of BPH • Medical Treatment with Alpha Blockers and 5 -Alpha Reductase Inhibitors are now established alternative to ‘Invasive Therapy’ Dr. Sunil Shroff - Dutasteride 2004

Clinical Progression of BPH Determines if a patient with BPH develops any of the below mentioned symptoms on follow up during watchful waiting Period ü AUR ü Recurrent urinary tract infection or urosepsis, ü Four-point or greater increase in baseline AUA symptom, ü Incontinence ü Need for prostate surgery ü Renal insufficiency due to BPH Dr. Sunil Shroff - Dutasteride 2004

BPH – Age Related Morbidity • Among 50 yrs old men, an estimated 35% lifetime incidence of surgical or medical intervention • A 60 years old has a 23% chance of experiencing Acute Urinary Retention if he survives for additional 20 years • Nearly 1 in 10 men in their 70’s will have Acute Osterling. JE. Benign. Retention prostatic hyperplasia: a review of its histogenesis 5 and natural history. Urinary in the subsequent years Prostate Suppl. 1996, 667 -73 Jacobson SJ et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997, 158: 481 -487 Dr. Sunil Shroff - Dutasteride 2004

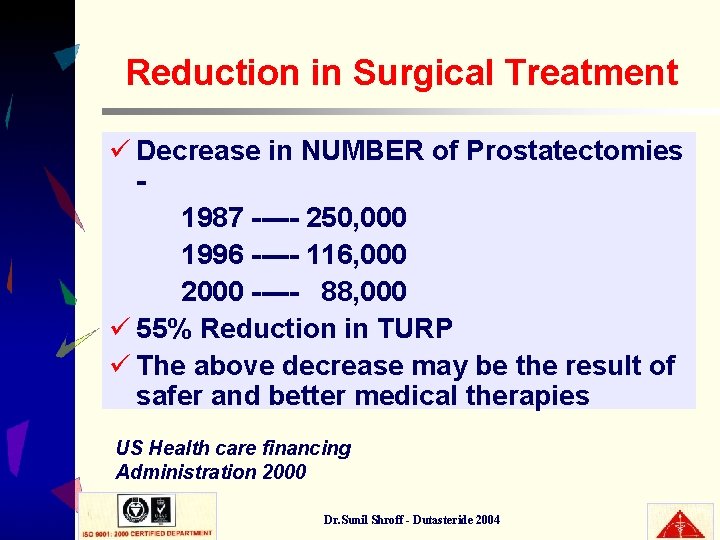
Reduction in Surgical Treatment ü Decrease in NUMBER of Prostatectomies 1987 ----- 250, 000 1996 ----- 116, 000 2000 ----- 88, 000 ü 55% Reduction in TURP ü The above decrease may be the result of safer and better medical therapies US Health care financing Administration 2000 Dr. Sunil Shroff - Dutasteride 2004

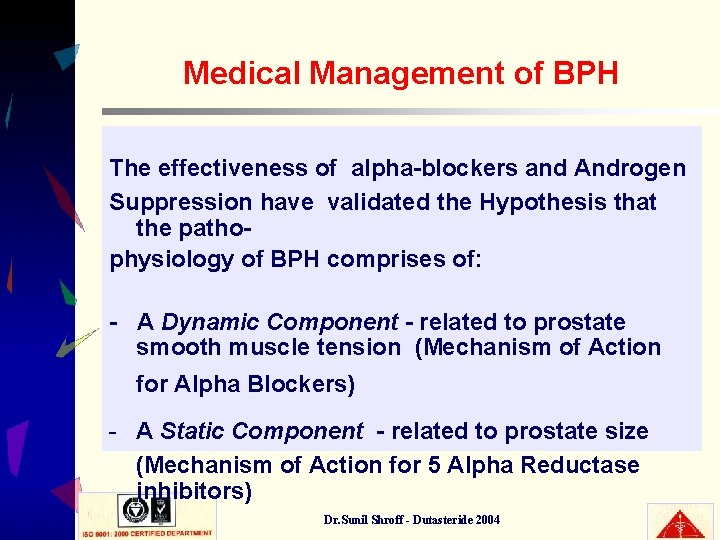
Medical Management of BPH The effectiveness of alpha-blockers and Androgen Suppression have validated the Hypothesis that the pathophysiology of BPH comprises of: - A Dynamic Component - related to prostate smooth muscle tension (Mechanism of Action for Alpha Blockers) - A Static Component - related to prostate size (Mechanism of Action for 5 Alpha Reductase inhibitors) Dr. Sunil Shroff - Dutasteride 2004

Does Prostate Volume Reduction Help • Prostate Volume does not have strong corelation to prostate symptoms • However Prostate volume is an important predictor of risk for developing acute urinary retention (AUR) • Finasteride - decreases the risk of progression to acute urinary retention benefit greatest in men with enlarged prostates Rev Urol. 2003; 5(suppl 5): S 28 -S 35]© 2003 Med Reviews Dr. Sunil Shroff - Dutasteride 2004

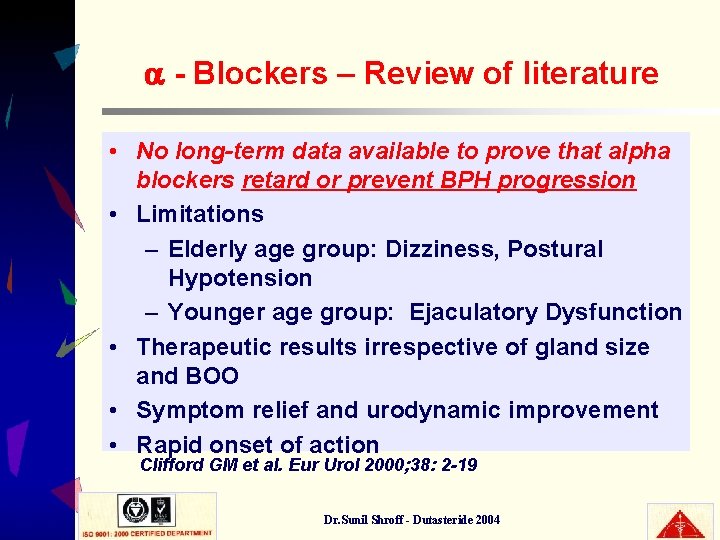
- Blockers – Review of literature • No long-term data available to prove that alpha blockers retard or prevent BPH progression • Limitations – Elderly age group: Dizziness, Postural Hypotension – Younger age group: Ejaculatory Dysfunction • Therapeutic results irrespective of gland size and BOO • Symptom relief and urodynamic improvement • Rapid onset of action Clifford GM et al. Eur Urol 2000; 38: 2 -19 Dr. Sunil Shroff - Dutasteride 2004

5 -Reductase Inhibitors: Review of literature • Reduce prostate volume • Reduce risk of progression to AUR • Reduce risk of prostatic surgery • Effective for long-term therapies • Improvement in Qo. L Clifford GM et al. Eur Urol 2000; 38: 2 -19 Dr. Sunil Shroff - Dutasteride 2004

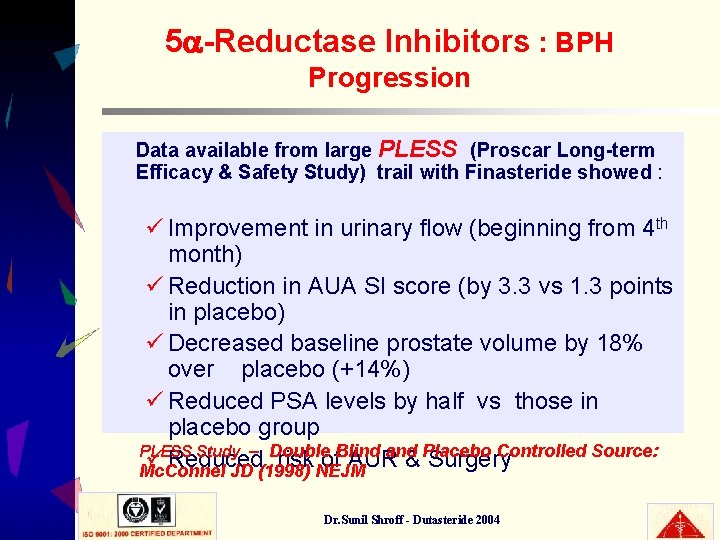
5 -Reductase Inhibitors : BPH Progression Data available from large PLESS (Proscar Long-term Efficacy & Safety Study) trail with Finasteride showed : ü Improvement in urinary flow (beginning from 4 th month) ü Reduction in AUA SI score (by 3. 3 vs 1. 3 points in placebo) ü Decreased baseline prostate volume by 18% over placebo (+14%) ü Reduced PSA levels by half vs those in placebo group PLESS Study – Double Blind and Placebo Controlled Source: ü Reduced risk of AUR & Surgery Mc. Connel JD (1998) NEJM Dr. Sunil Shroff - Dutasteride 2004

Dutasteride – Initial Indian Experience • Dutasteride - Drug Profile • Dutasteride – Results of Seven Centres • Dutasteride – Comparison and Discussion Testosterone Dihydrotestostero 5α-Reductase DHT is the androgen primarily responsible for the initial development and subsequent enlargement of the prostate gland. Dutasteride inhibits the conversion of testosterone to Dihydrotestosterone (DHT) Dr. Sunil Shroff - Dutasteride 2004

Dutasteride - History of Drug • Dutasteride – Filed for treatment of BPH in 1995 • Dutasteride Approved by FDA on 20 th Nov 2001 • Dutasteride Now available in India – Dr. Reddys Dr. Sunil Shroff - Dutasteride 2004

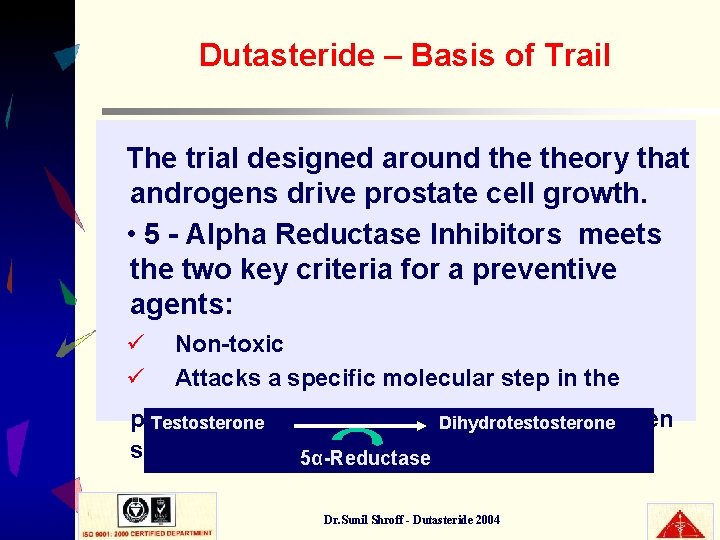
Dutasteride – Basis of Trail The trial designed around theory that androgens drive prostate cell growth. • 5 - Alpha Reductase Inhibitors meets the two key criteria for a preventive agents: ü ü Non-toxic Attacks a specific molecular step in the prostatic tissue to selectively achieve androgen Testosterone Dihydrotestosterone suppression 5α-Reductase Dr. Sunil Shroff - Dutasteride 2004

5α-Reductase Testosterone is converted to DHT by the enzyme 5α reductase, which exists as Two Isoforms: Type I and Type II – The type I Isoenzyme is also responsible for testosterone conversion in the Skin and Liver. – The type II - Isoenzyme is primarily active in the Reproductive Tissues Dutasteride a novel dual 5α-Reductase inhibitor Dr. Sunil Shroff - Dutasteride 2004

Dutasteride – A Novel 5 -ARI • 4 - azasteroid • Selective and potent inhibitor of both type I & II 5 -AR • Unlike Finasteride, inhibits: *Type I - 5 AR : 45 fold *Type II -5 AR : 2. 5 fold • 5 times more rapid onset of action Source : Drugs Ageing ( 2003) Dr. Sunil Shroff - Dutasteride 2004

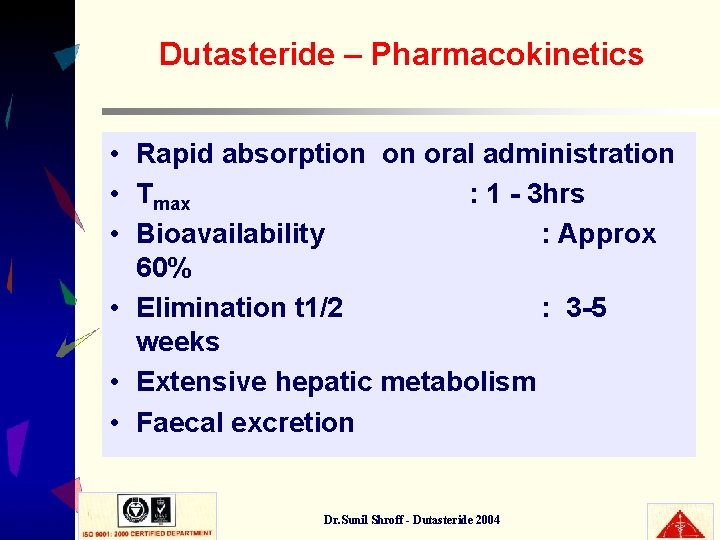
Dutasteride – Pharmacokinetics • Rapid absorption on oral administration • Tmax : 1 - 3 hrs • Bioavailability : Approx 60% • Elimination t 1/2 : 3 -5 weeks • Extensive hepatic metabolism • Faecal excretion Dr. Sunil Shroff - Dutasteride 2004

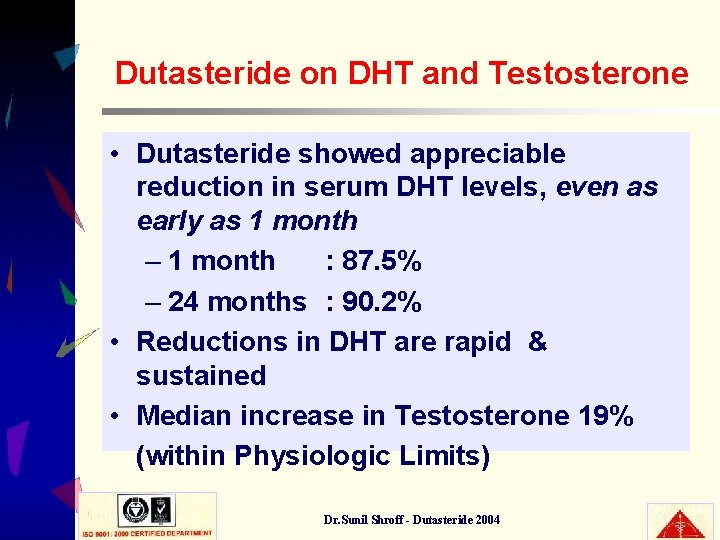
Dutasteride on DHT and Testosterone • Dutasteride showed appreciable reduction in serum DHT levels, even as early as 1 month – 1 month : 87. 5% – 24 months : 90. 2% • Reductions in DHT are rapid & sustained • Median increase in Testosterone 19% (within Physiologic Limits) Dr. Sunil Shroff - Dutasteride 2004

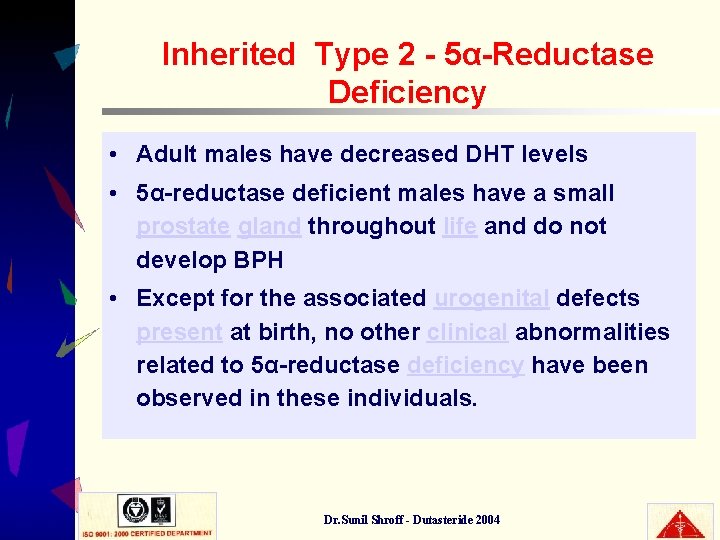
Inherited Type 2 - 5α-Reductase Deficiency • Adult males have decreased DHT levels • 5α-reductase deficient males have a small prostate gland throughout life and do not develop BPH • Except for the associated urogenital defects present at birth, no other clinical abnormalities related to 5α-reductase deficiency have been observed in these individuals. Dr. Sunil Shroff - Dutasteride 2004

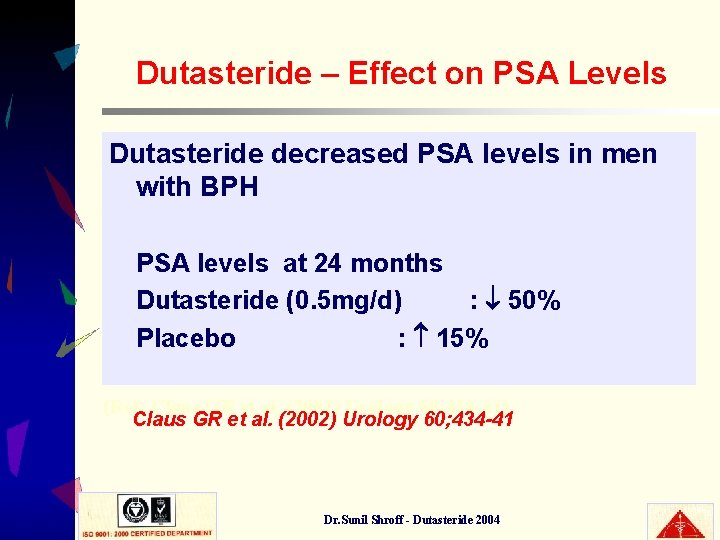
Dutasteride – Effect on PSA Levels Dutasteride decreased PSA levels in men with BPH PSA levels at 24 months Dutasteride (0. 5 mg/d) : 50% Placebo : 15% (Ref: Claus GR et al. (2002) Urology 60; 434 -41) Claus GR et al. (2002) Urology 60; 434 -41 Dr. Sunil Shroff - Dutasteride 2004

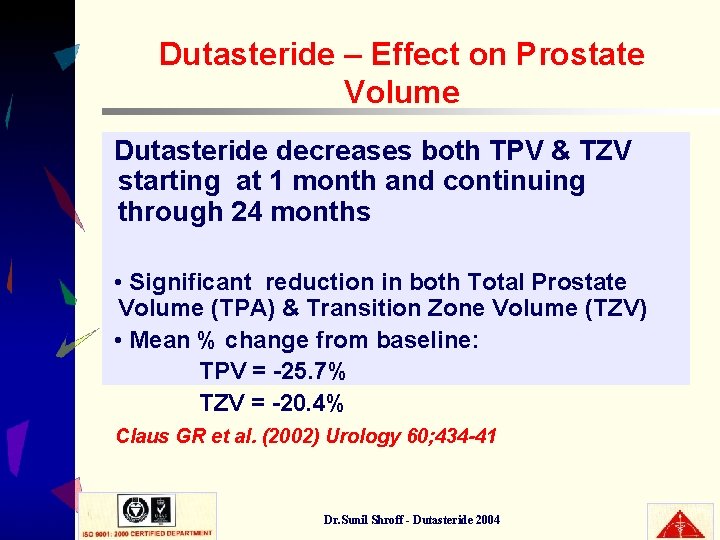
Dutasteride – Effect on Prostate Volume Dutasteride decreases both TPV & TZV starting at 1 month and continuing through 24 months • Significant reduction in both Total Prostate Volume (TPA) & Transition Zone Volume (TZV) • Mean % change from baseline: TPV = -25. 7% TZV = -20. 4% Claus GR et al. (2002) Urology 60; 434 -41 Dr. Sunil Shroff - Dutasteride 2004

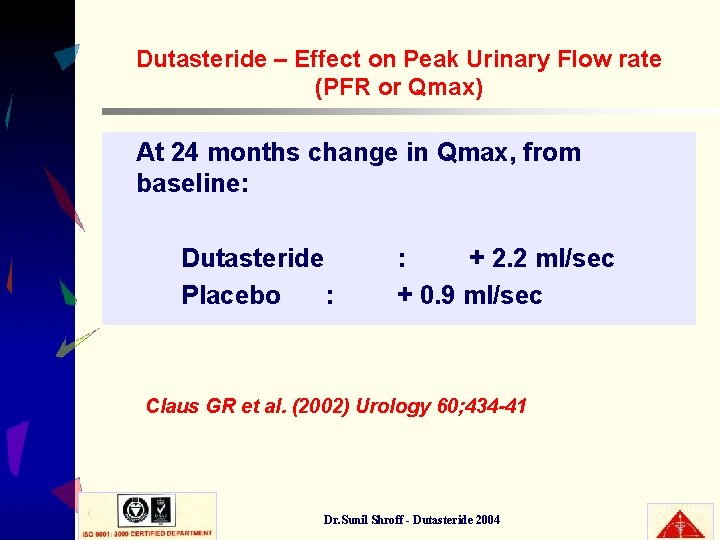
Dutasteride – Effect on Peak Urinary Flow rate (PFR or Qmax) At 24 months change in Qmax, from baseline: Dutasteride Placebo : : + 2. 2 ml/sec + 0. 9 ml/sec Claus GR et al. (2002) Urology 60; 434 -41 Dr. Sunil Shroff - Dutasteride 2004

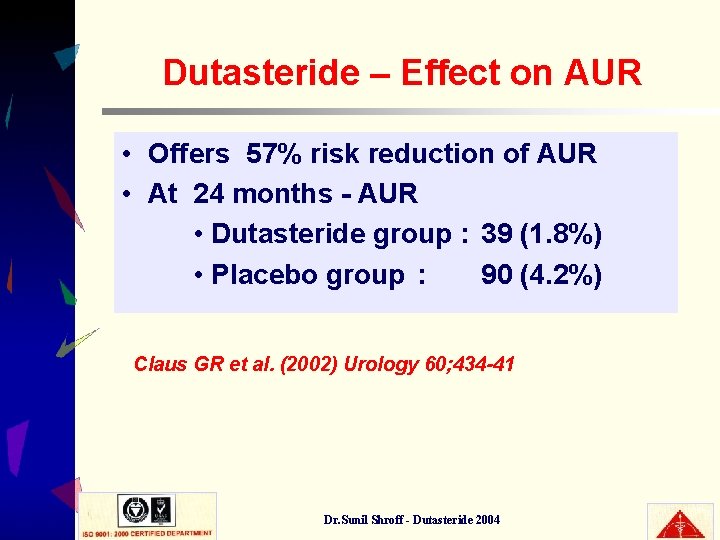
Dutasteride – Effect on AUR • Offers 57% risk reduction of AUR • At 24 months - AUR • Dutasteride group : 39 (1. 8%) • Placebo group : 90 (4. 2%) Claus GR et al. (2002) Urology 60; 434 -41 Dr. Sunil Shroff - Dutasteride 2004

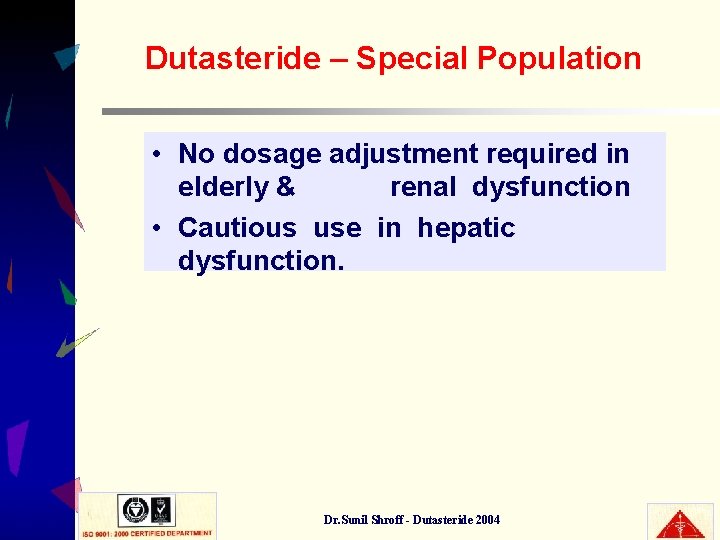
Dutasteride – Special Population • No dosage adjustment required in elderly & renal dysfunction • Cautious use in hepatic dysfunction. Dr. Sunil Shroff - Dutasteride 2004

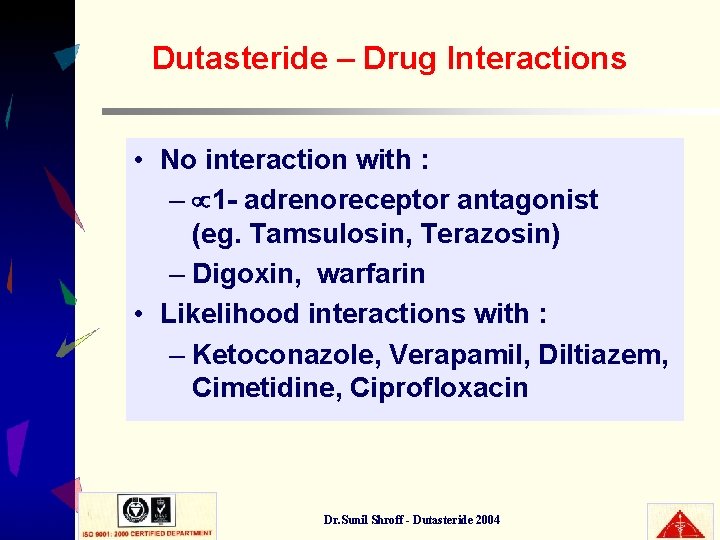
Dutasteride – Drug Interactions • No interaction with : – 1 - adrenoreceptor antagonist (eg. Tamsulosin, Terazosin) – Digoxin, warfarin • Likelihood interactions with : – Ketoconazole, Verapamil, Diltiazem, Cimetidine, Ciprofloxacin Dr. Sunil Shroff - Dutasteride 2004

Metabolism and Elimination • Dutasteride is extensively metabolized in humans • Dutasteride and its metabolites are excreted mainly in feces. • Only trace amounts of unchanged Dutasteride can be found in urine (<1%). Dr. Sunil Shroff - Dutasteride 2004

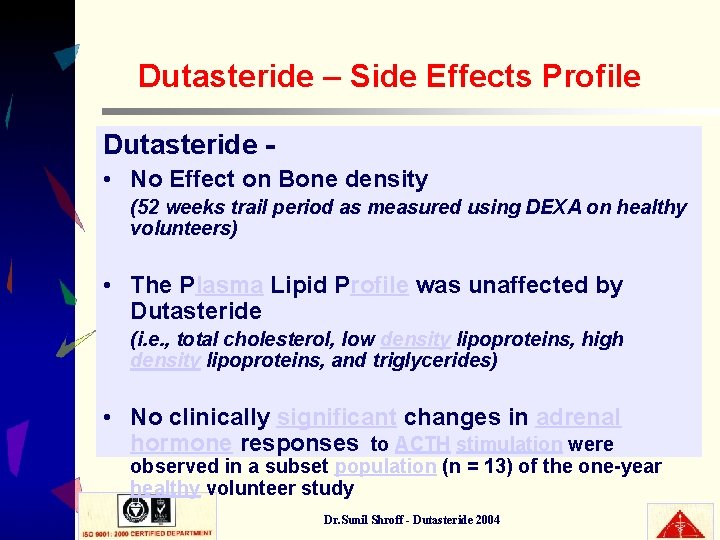
Dutasteride – Side Effects Profile Dutasteride • No Effect on Bone density (52 weeks trail period as measured using DEXA on healthy volunteers) • The Plasma Lipid Profile was unaffected by Dutasteride (i. e. , total cholesterol, low density lipoproteins, high density lipoproteins, and triglycerides) • No clinically significant changes in adrenal hormone responses to ACTH stimulation were observed in a subset population (n = 13) of the one-year healthy volunteer study Dr. Sunil Shroff - Dutasteride 2004

DUTAS (Dutasteride) The Indian Experience Dr. Sunil Shroff - Dutasteride 2004

DUTAS (Dutasteride) An open prospective Phase III study to evaluate The efficacy & safety of Dutasteride in men with Benign Prostatic Hyperplasia (BPH) Dr. Sunil Shroff - Dutasteride 2004

Clinical Trial Setting Seven Centres St. John’s Medical College, Bangalore Nizam’s Institute of Med Sci. , Hyderabad M. S. Ramaiah Hospital, Bangalore PSG Institute of Medical Sciences, Coimbatore Nair Hospital , Mumbai Sri Ramachandra Medical College , Chennai Care Hospital, Hyderabad After approval of Institutional Ethics Commitee and informed co Dr. Sunil Shroff - Dutasteride 2004

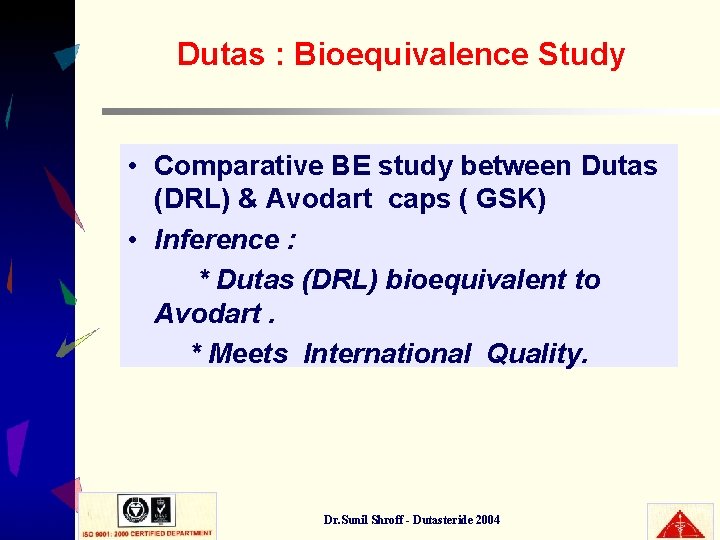
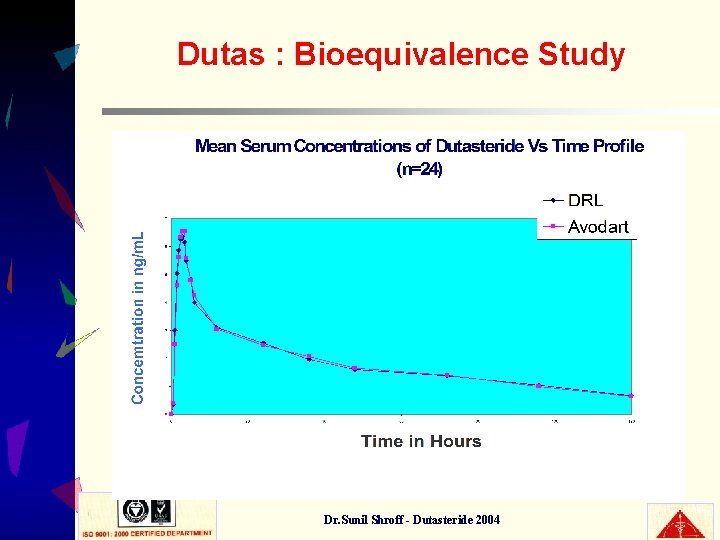
Dutas : Bioequivalence Study • Comparative BE study between Dutas (DRL) & Avodart caps ( GSK) • Inference : * Dutas (DRL) bioequivalent to Avodart. * Meets International Quality. Dr. Sunil Shroff - Dutasteride 2004

Dutas : Bioequivalence Study Dr. Sunil Shroff - Dutasteride 2004

Prescribing Information : Dutasteride Composition: Each soft gelatin capsule contains: Dutasteride O. 5 mg Capsules should be swallowed whole Colour: Iron Oxide Black and Iron Oxide Red Warnings & Precautions: • Women who are pregnant or may become pregnant should not handle Dutasteride capsules because of possible absorption thro’ skin and risk of anomaly to male foetus. • Caution should be exercised in patients with liver Dr. Sunil Shroff - Dutasteride 2004 disease

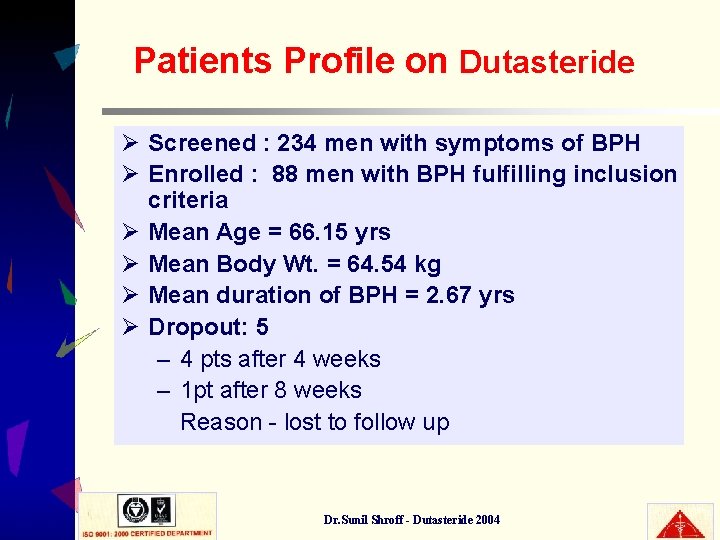
Patients Profile on Dutasteride Ø Screened : 234 men with symptoms of BPH Ø Enrolled : 88 men with BPH fulfilling inclusion criteria Ø Mean Age = 66. 15 yrs Ø Mean Body Wt. = 64. 54 kg Ø Mean duration of BPH = 2. 67 yrs Ø Dropout: 5 – 4 pts after 4 weeks – 1 pt after 8 weeks Reason - lost to follow up Dr. Sunil Shroff - Dutasteride 2004

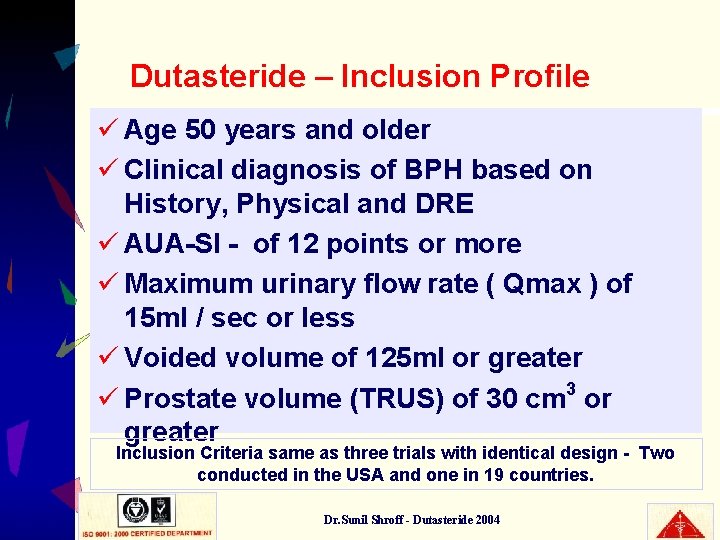
Dutasteride – Inclusion Profile ü Age 50 years and older ü Clinical diagnosis of BPH based on History, Physical and DRE ü AUA-SI - of 12 points or more ü Maximum urinary flow rate ( Qmax ) of 15 ml / sec or less ü Voided volume of 125 ml or greater ü Prostate volume (TRUS) of 30 cm 3 or greater Inclusion Criteria same as three trials with identical design - Two conducted in the USA and one in 19 countries. Dr. Sunil Shroff - Dutasteride 2004

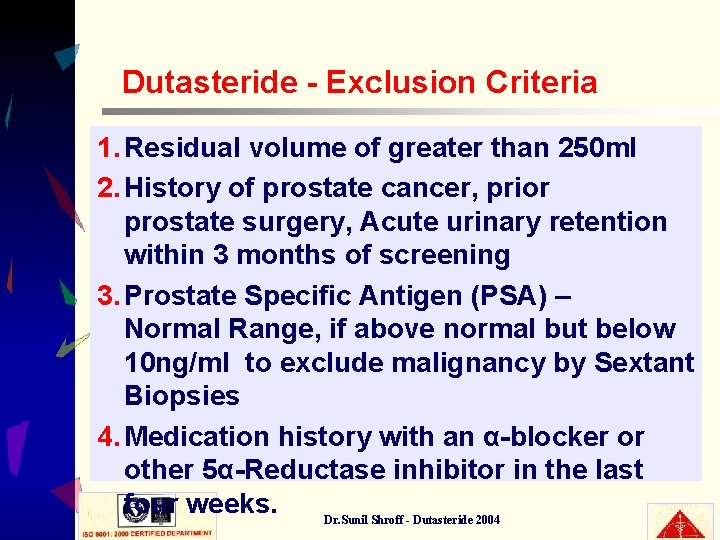
Dutasteride - Exclusion Criteria 1. Residual volume of greater than 250 ml 2. History of prostate cancer, prior prostate surgery, Acute urinary retention within 3 months of screening 3. Prostate Specific Antigen (PSA) – Normal Range, if above normal but below 10 ng/ml to exclude malignancy by Sextant Biopsies 4. Medication history with an α-blocker or other 5α-Reductase inhibitor in the last four weeks. Dr. Sunil Shroff - Dutasteride 2004

Evaluation Parameters ü AUA - SI - ( Week 0, 4, 8 and 12 ) ü TPV (TRUS) - ( Week 0, 4 and 12 ) ü Qmax - ( Week 0, 4 and 12 ) ü PSA - ( Week 0 and 12 ) ü Clinical Adverse Event - ( Week 0 – 12 ) ü Hematological and Biochemical Adverse Events - ( Week 0 and 12 ) Dr. Sunil Shroff - Dutasteride 2004

DUTAS Dutasteride RESULTS Dr. Sunil Shroff - Dutasteride 2004

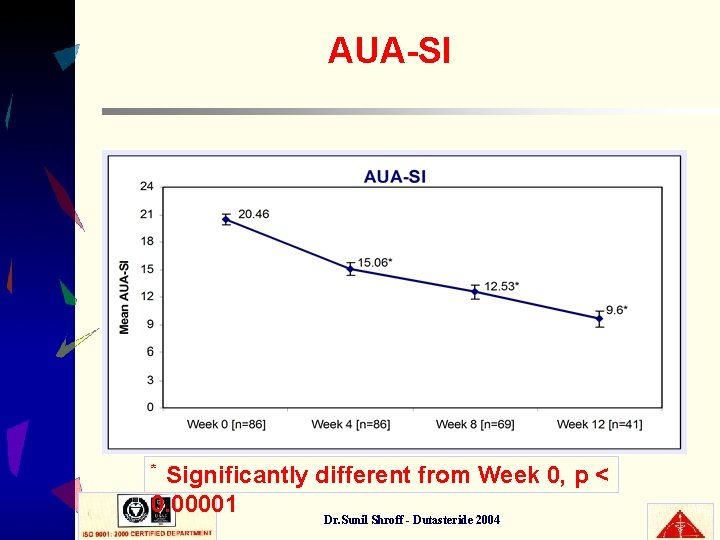
AUA-SI Significantly different from Week 0, p < 0. 00001 * Dr. Sunil Shroff - Dutasteride 2004

Total Prostate Volume Significantly different from Week 0, p < 0. 00001 * Dr. Sunil Shroff - Dutasteride 2004

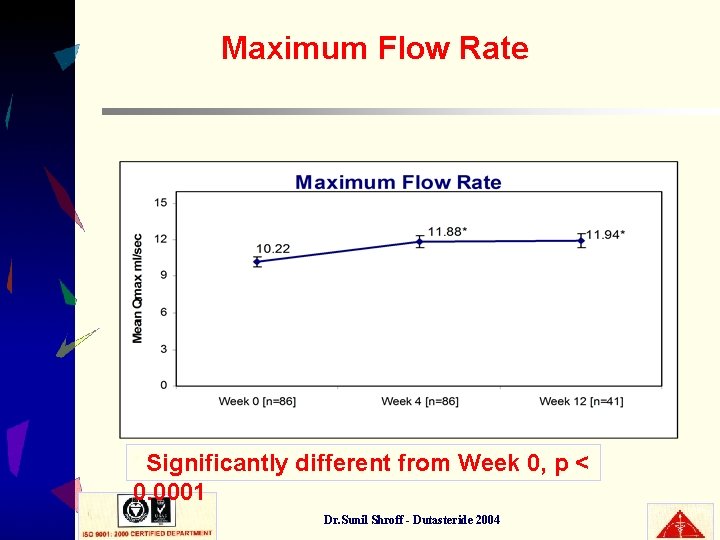
Maximum Flow Rate Significantly different from Week 0, p < 0. 0001 * Dr. Sunil Shroff - Dutasteride 2004

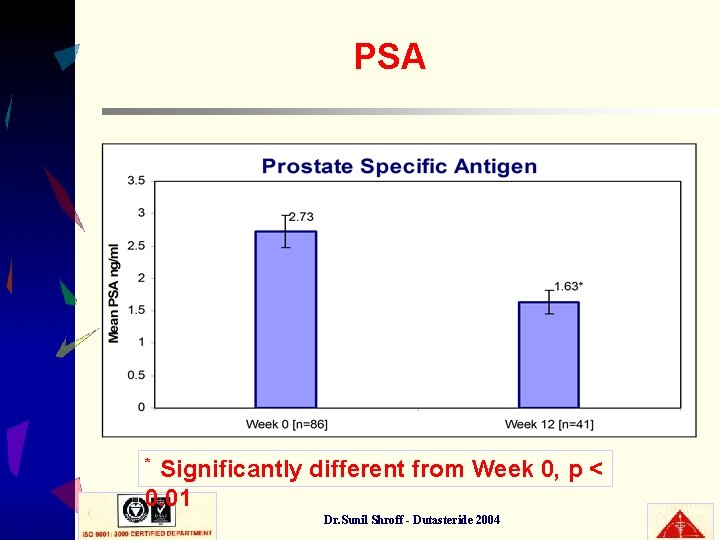
PSA Significantly different from Week 0, p < 0. 01 * Dr. Sunil Shroff - Dutasteride 2004

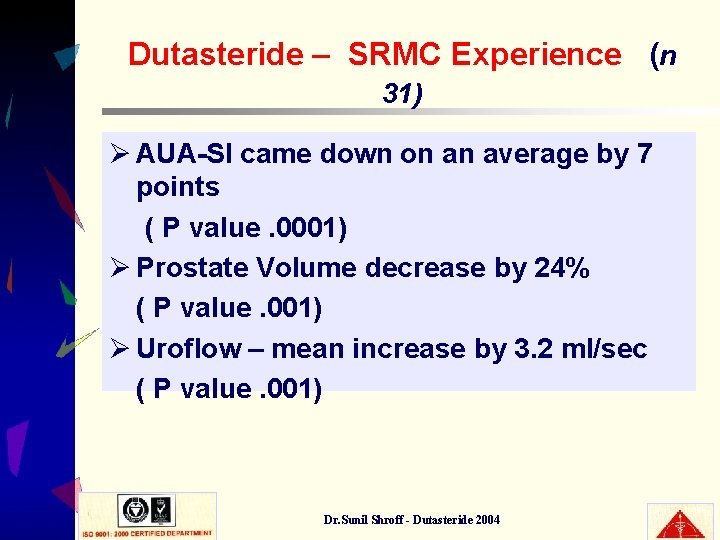
Dutasteride – SRMC Experience (n 31) Ø AUA-SI came down on an average by 7 points ( P value. 0001) Ø Prostate Volume decrease by 24% ( P value. 001) Ø Uroflow – mean increase by 3. 2 ml/sec ( P value. 001) Dr. Sunil Shroff - Dutasteride 2004

Drug Safety Seven patients reported adverse events • Loss of libido (3), • Erectile dysfunction (3) • Abdominal pain (1) • One patient with edema had to discontinue therapy due to concomitant cardiovascular disorder. • No significant changes in hematological and biochemical parameters at week 12 compared to baseline Dr. Sunil Shroff - Dutasteride 2004

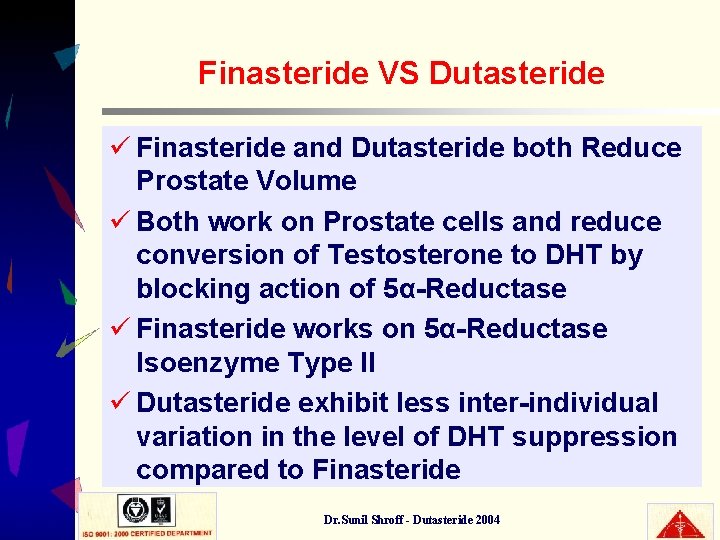
Finasteride VS Dutasteride ü Finasteride and Dutasteride both Reduce Prostate Volume ü Both work on Prostate cells and reduce conversion of Testosterone to DHT by blocking action of 5α-Reductase ü Finasteride works on 5α-Reductase Isoenzyme Type II ü Dutasteride exhibit less inter-individual variation in the level of DHT suppression compared to Finasteride Dr. Sunil Shroff - Dutasteride 2004

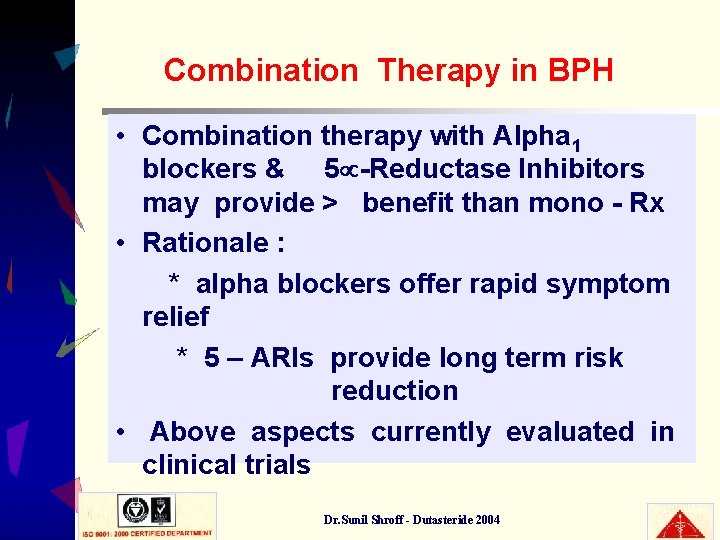
Combination Therapy in BPH • Combination therapy with Alpha 1 blockers & 5 -Reductase Inhibitors may provide > benefit than mono - Rx • Rationale : * alpha blockers offer rapid symptom relief * 5 – ARIs provide long term risk reduction • Above aspects currently evaluated in clinical trials Dr. Sunil Shroff - Dutasteride 2004

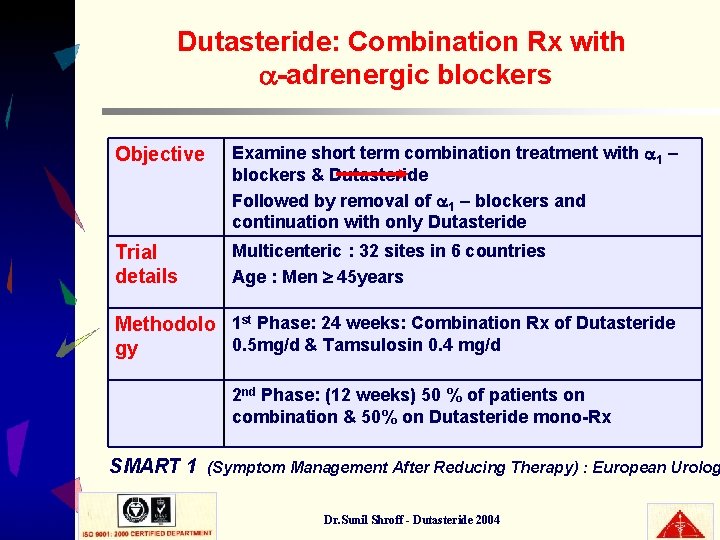
Dutasteride: Combination Rx with -adrenergic blockers Objective Examine short term combination treatment with 1 – blockers & Dutasteride Followed by removal of 1 – blockers and continuation with only Dutasteride Trial details Multicenteric : 32 sites in 6 countries Age : Men 45 years Methodolo 1 st Phase: 24 weeks: Combination Rx of Dutasteride 0. 5 mg/d & Tamsulosin 0. 4 mg/d gy 2 nd Phase: (12 weeks) 50 % of patients on combination & 50% on Dutasteride mono-Rx SMART 1 (Symptom Management After Reducing Therapy) : European Urolog Dr. Sunil Shroff - Dutasteride 2004

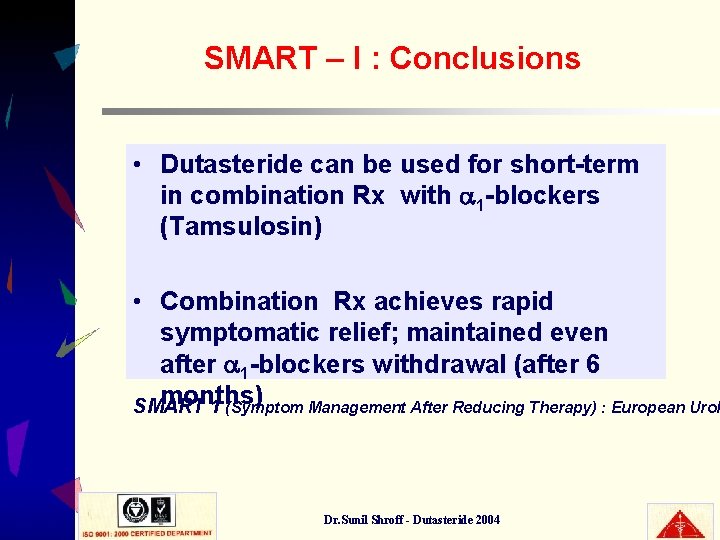
SMART – I : Conclusions • Dutasteride can be used for short-term in combination Rx with 1 -blockers (Tamsulosin) • Combination Rx achieves rapid symptomatic relief; maintained even after 1 -blockers withdrawal (after 6 months) SMART 1 (Symptom Management After Reducing Therapy) : European Urol Dr. Sunil Shroff - Dutasteride 2004

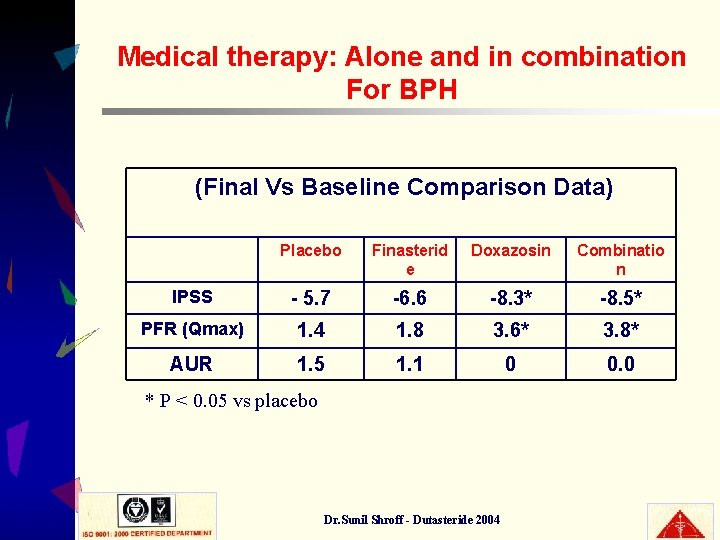
Medical therapy: Alone and in combination For BPH (Final Vs Baseline Comparison Data) Placebo Finasterid e Doxazosin Combinatio n IPSS - 5. 7 -6. 6 -8. 3* -8. 5* PFR (Qmax) 1. 4 1. 8 3. 6* 3. 8* AUR 1. 5 1. 1 0 0. 0 * P < 0. 05 vs placebo Dr. Sunil Shroff - Dutasteride 2004

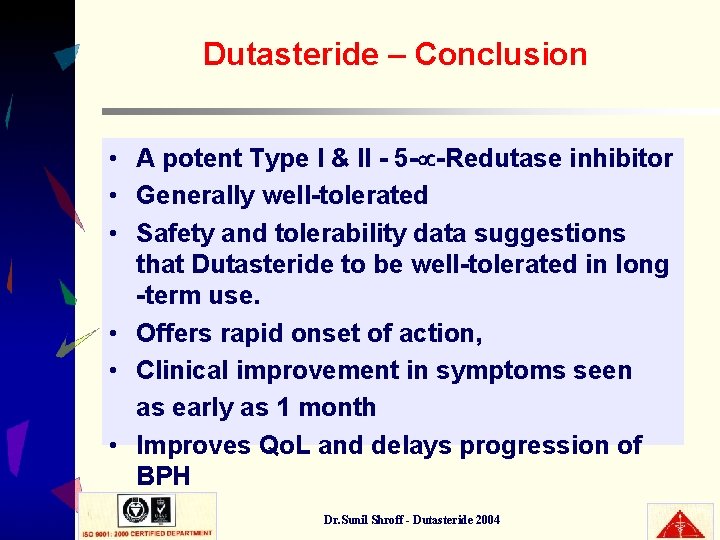
Dutasteride – Conclusion • A potent Type I & II - 5 - -Redutase inhibitor • Generally well-tolerated • Safety and tolerability data suggestions that Dutasteride to be well-tolerated in long -term use. • Offers rapid onset of action, • Clinical improvement in symptoms seen as early as 1 month • Improves Qo. L and delays progression of BPH Dr. Sunil Shroff - Dutasteride 2004

Dutasteride – Conclusion • 45 and 2. 5 times more potent in inhibiting type I & II – 5 AR respectively than Finasteride • Can be given effectively in combination with 1 – adrenergic blockers • Pilot Indian experience in phase 3 trials, matches with International data. • Larger multi-centre study can substantiate the above conclusion in Indian Patients Dr. Sunil Shroff - Dutasteride 2004

References - Dutasteride 1. Osterling. JE. Benign Prostatic Hyperplasia: A Review of its Histogenesis and Natural History. Prostate Suppl. 1996, 667 -73 2. Jacobson S J et al. Natural History of Prostatism: Risk Factors for Acute Urinary Retention 3. J Urol. 1997, 158: 481 -487 4. US Health care Financing Administration 2000 5. Rev Urol. 2003; 5 (suppl 5): S 28 -S 35] © 2003 Med Reviews 6. Clifford GM et al. Eur Urol 2000; 38: 2 -19 7. PLESS Study – Double Blind and Placebo Controlled 8. Mc. Connel JD (1998) NEJM 9. Claus GR et al. (2002) Urology 60; 434 -41 10. SMART 1 (Symptom Management After Reducing Therapy) : European Urology, 2003 11. PREDICT: Kirby et al J Urol 1999; 161; 266 Dr. Sunil Shroff - Dutasteride 2004

Dutasteride – Indian Experience Sunil Shroff Sri Ramachandra Hospital Chennai sshroff@vsnl. com THANK YOU Dr. Sunil Shroff - Dutasteride 2004