Duration of DAPT Post DAPT Trial Laura Mauri



- Slides: 16

Duration of DAPT Post DAPT Trial Laura Mauri, M. D. , M. Sc. Brigham and Women’s Hospital Harvard Medical School Boston, MA 1

Disclosures - Laura Mauri, M. D. , M. Sc. • Research grants from Abbott, Boston Scientific, Cordis, Medtronic, Eli Lilly, Daiichi Sankyo, Bristol-Myers Squibb, Biotronik, and Sanofi-Aventis • Consulting Medtronic, Eli Lilly, Biotronik, Re. Cor, St. Jude 2

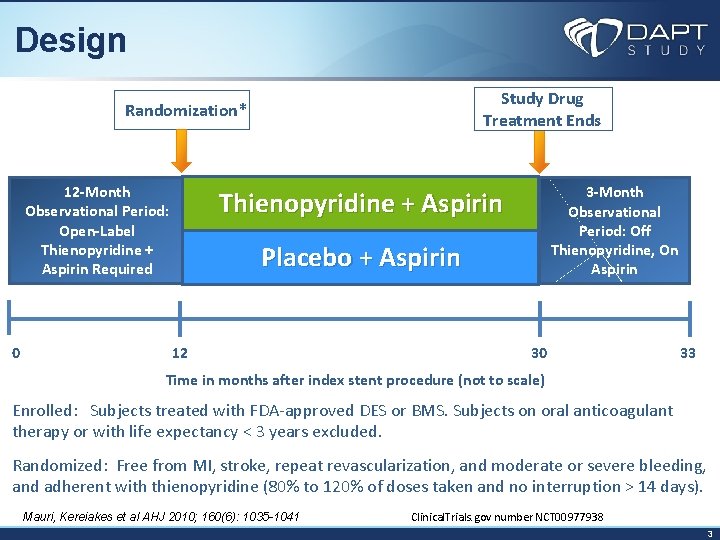
Design Study Drug Treatment Ends Randomization* 12 -Month Observational Period: Open-Label Thienopyridine + Aspirin Required 0 3 -Month Observational Period: Off Thienopyridine, On Aspirin Thienopyridine + Aspirin Placebo + Aspirin 12 30 33 Time in months after index stent procedure (not to scale) Enrolled: Subjects treated with FDA-approved DES or BMS. Subjects on oral anticoagulant therapy or with life expectancy < 3 years excluded. Randomized: Free from MI, stroke, repeat revascularization, and moderate or severe bleeding, and adherent with thienopyridine (80% to 120% of doses taken and no interruption > 14 days). Mauri, Kereiakes et al AHJ 2010; 160(6): 1035 -1041 Clinical. Trials. gov number NCT 00977938 3

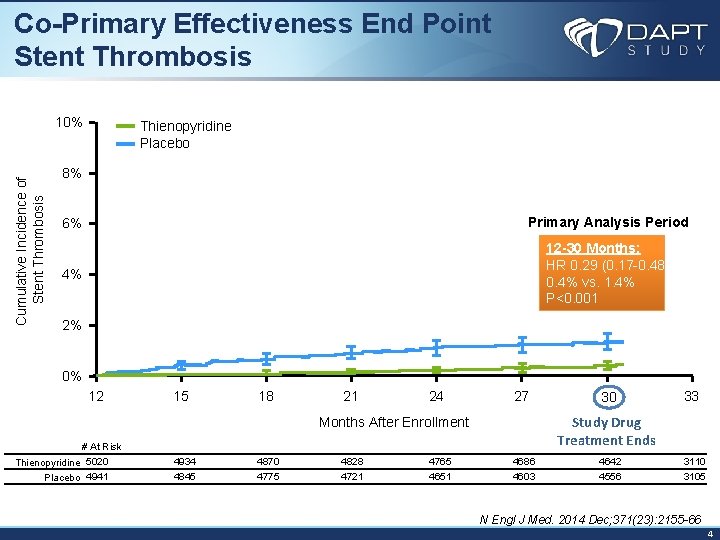
Co-Primary Effectiveness End Point Stent Thrombosis Cumulative Incidence of Stent Thrombosis 10% Thienopyridine Placebo 8% 6% Primary Analysis Period 4% 12 -30 Months: HR 0. 29 (0. 17 -0. 48) 0. 4% vs. 1. 4% P<0. 001 2% 0% 12 15 18 21 24 27 33 Study Drug Treatment Ends Months After Enrollment # At Risk Thienopyridine 5020 Placebo 4941 30 4934 4870 4828 4765 4686 4642 3110 4845 4775 4721 4651 4603 4556 3105 N Engl J Med. 2014 Dec; 371(23): 2155 -66 4

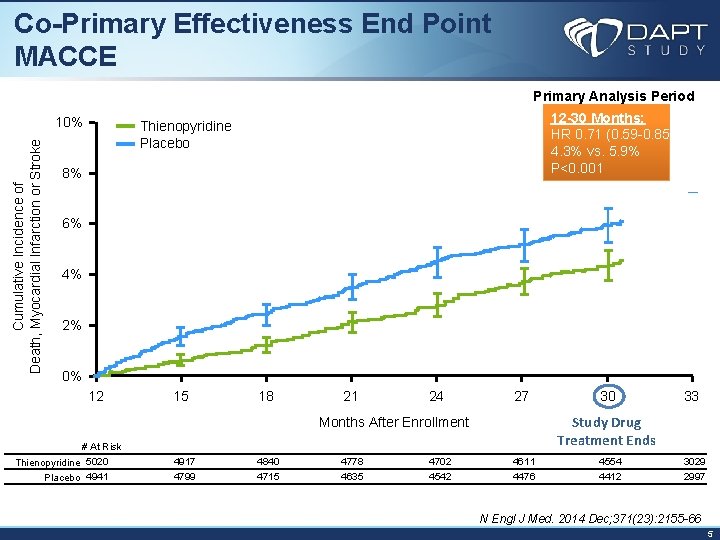
Co-Primary Effectiveness End Point MACCE Primary Analysis Period Cumulative Incidence of Death, Myocardial Infarction or Stroke 10% 12 -30 Months: HR 0. 71 (0. 59 -0. 85) 4. 3% vs. 5. 9% P<0. 001 Thienopyridine Placebo 8% 6% 4% 2% 0% 12 15 18 21 24 27 33 Study Drug Treatment Ends Months After Enrollment # At Risk Thienopyridine 5020 Placebo 4941 30 4917 4840 4778 4702 4611 4554 3029 4799 4715 4635 4542 4476 4412 2997 N Engl J Med. 2014 Dec; 371(23): 2155 -66 5

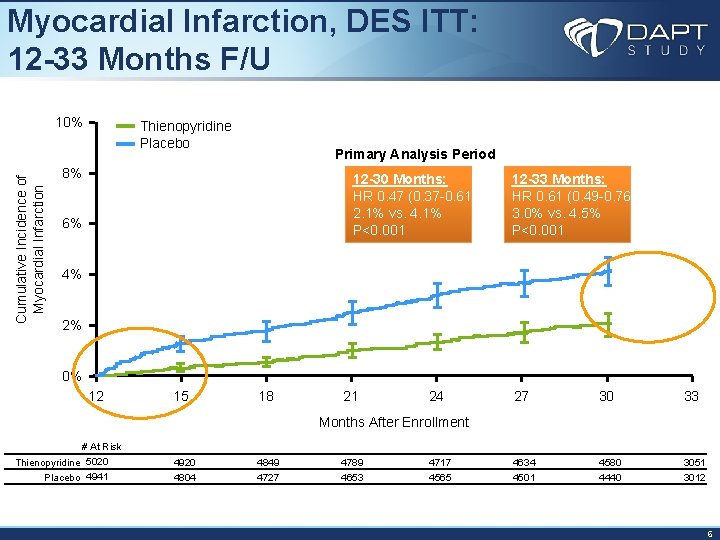
Myocardial Infarction, DES ITT: 12 -33 Months F/U Cumulative Incidence of Myocardial Infarction 10% Thienopyridine Placebo Primary Analysis Period 8% 12 -30 Months: HR 0. 47 (0. 37 -0. 61) 2. 1% vs. 4. 1% P<0. 001 6% 12 -33 Months: HR 0. 61 (0. 49 -0. 76) 3. 0% vs. 4. 5% P<0. 001 4% 2% 0% 12 15 18 21 24 27 30 33 Months After Enrollment # At Risk Thienopyridine 5020 Placebo 4941 4920 4849 4789 4717 4634 4580 3051 4804 4727 4653 4565 4501 4440 3012 6

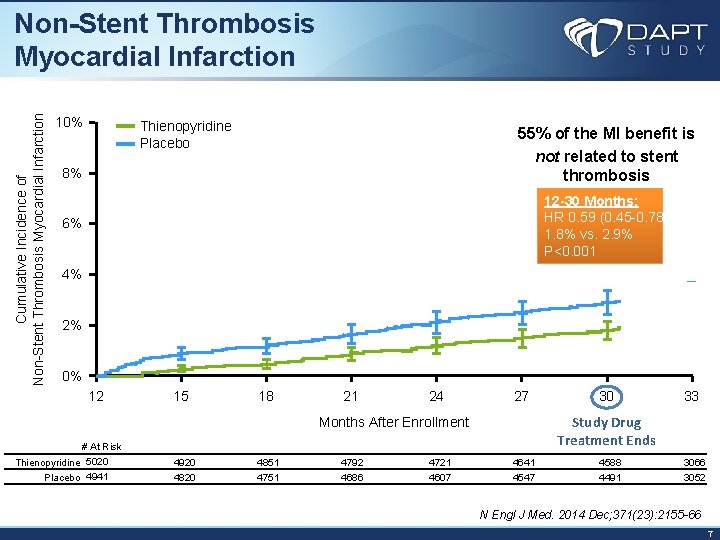
Cumulative Incidence of Non-Stent Thrombosis Myocardial Infarction 10% Thienopyridine Placebo 55% of the MI benefit is not related to stent thrombosis 8% 12 -30 Months: HR 0. 59 (0. 45 -0. 78) 1. 8% vs. 2. 9% P<0. 001 6% 4% 2% 0% 12 15 18 21 24 27 33 Study Drug Treatment Ends Months After Enrollment # At Risk Thienopyridine 5020 Placebo 4941 30 4920 4851 4792 4721 4641 4588 3066 4820 4751 4686 4607 4547 4491 3052 N Engl J Med. 2014 Dec; 371(23): 2155 -66 7

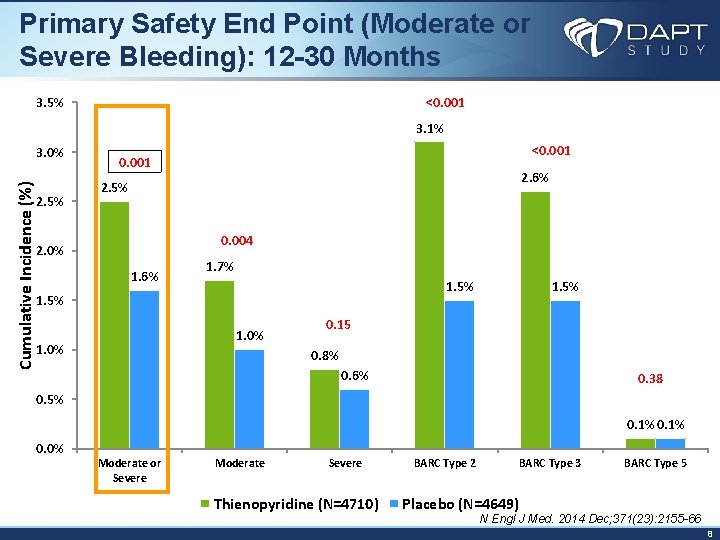
Primary Safety End Point (Moderate or Severe Bleeding): 12 -30 Months <0. 001 3. 5% 3. 1% Cumulative Incidence (%) 3. 0% 2. 5% <0. 001 2. 6% 2. 5% 0. 004 2. 0% 1. 6% 1. 7% 1. 5% 1. 0% 1. 5% 0. 15 0. 8% 0. 6% 0. 38 0. 5% 0. 1% 0. 0% Moderate or Severe Moderate Severe Thienopyridine (N=4710) BARC Type 2 BARC Type 3 BARC Type 5 Placebo (N=4649) N Engl J Med. 2014 Dec; 371(23): 2155 -66 8

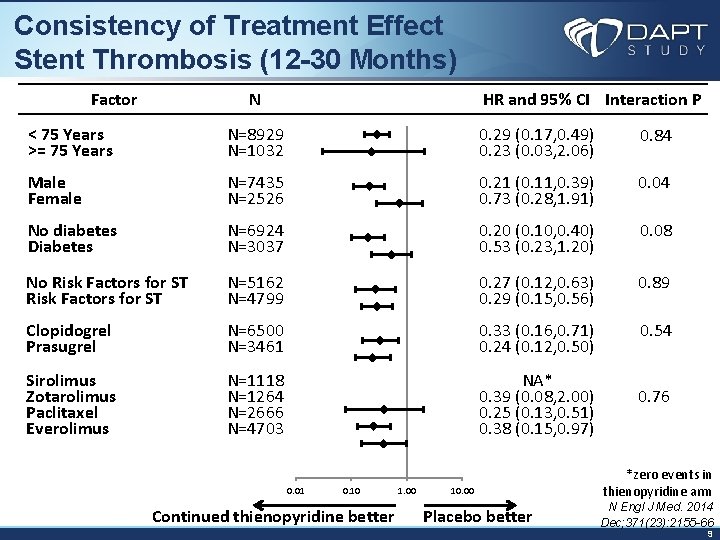
Consistency of Treatment Effect Stent Thrombosis (12 -30 Months) Factor N < 75 Years >= 75 Years N=8929 N=1032 Male Female N=7435 N=2526 No diabetes Diabetes N=6924 N=3037 No Risk Factors for ST N=5162 N=4799 Clopidogrel Prasugrel Sirolimus Zotarolimus Paclitaxel Everolimus HR and 95% CI Interaction P 0. 29 (0. 17, 0. 49) 0. 23 (0. 03, 2. 06) 0. 21 (0. 11, 0. 39) 0. 73 (0. 28, 1. 91) 0. 84 0. 04 0. 89 N=6500 N=3461 0. 20 (0. 10, 0. 40) 0. 53 (0. 23, 1. 20) 0. 27 (0. 12, 0. 63) 0. 29 (0. 15, 0. 56) 0. 33 (0. 16, 0. 71) 0. 24 (0. 12, 0. 50) N=1118 N=1264 N=2666 N=4703 NA* 0. 39 (0. 08, 2. 00) 0. 25 (0. 13, 0. 51) 0. 38 (0. 15, 0. 97) 0. 76 0. 01 0. 10 Continued thienopyridine better 1. 00 10. 00 Placebo better 0. 08 0. 54 *zero events in thienopyridine arm N Engl J Med. 2014 Dec; 371(23): 2155 -66 9

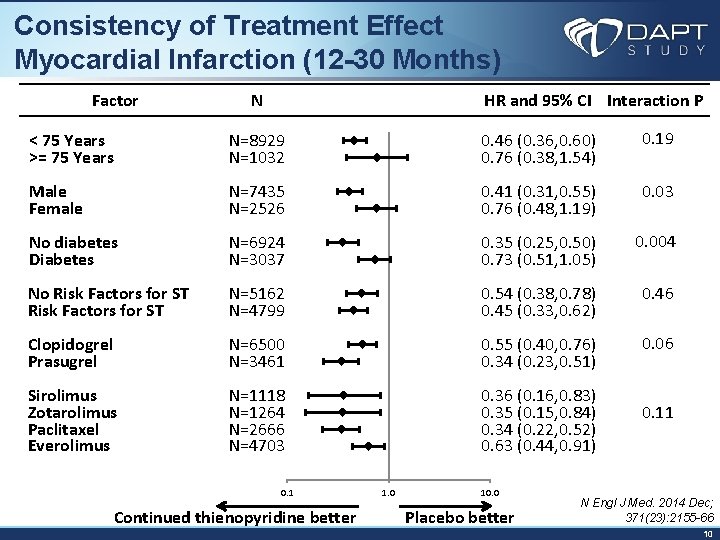
Consistency of Treatment Effect Myocardial Infarction (12 -30 Months) Factor N < 75 Years >= 75 Years N=8929 N=1032 Male Female N=7435 N=2526 No diabetes Diabetes N=6924 N=3037 No Risk Factors for ST N=5162 N=4799 Clopidogrel Prasugrel N=6500 N=3461 Sirolimus Zotarolimus Paclitaxel Everolimus N=1118 N=1264 N=2666 N=4703 0. 1 Continued thienopyridine better HR and 95% CI Interaction P 0. 19 0. 46 (0. 36, 0. 60) 0. 76 (0. 38, 1. 54) 0. 03 0. 41 (0. 31, 0. 55) 0. 76 (0. 48, 1. 19) 0. 004 0. 35 (0. 25, 0. 50) 0. 73 (0. 51, 1. 05) 0. 46 0. 54 (0. 38, 0. 78) 0. 45 (0. 33, 0. 62) 0. 06 0. 55 (0. 40, 0. 76) 0. 34 (0. 23, 0. 51) 0. 36 (0. 16, 0. 83) 0. 35 (0. 15, 0. 84) 0. 11 0. 34 (0. 22, 0. 52) 0. 63 (0. 44, 0. 91) 1. 0 10. 0 Placebo better N Engl J Med. 2014 Dec; 371(23): 2155 -66 10

• Continued thienopyridine therapy reduces stent thrombosis and non stent related myocardial infarction beyond 1 year after PCI, across drugs, stents, patient types • These findings offer an additional tool for secondary prevention in patients with coronary artery disease • While moderate bleeding is increased with continued DAPT, among patients who tolerate 1 year of DAPT, continuation of therapy provides an overall benefit 11

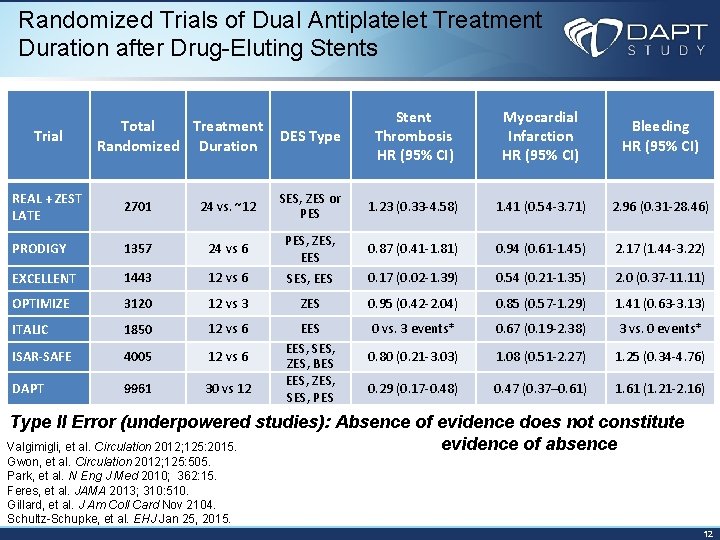
Randomized Trials of Dual Antiplatelet Treatment Duration after Drug-Eluting Stents Trial Total Treatment Randomized Duration DES Type Stent Thrombosis HR (95% CI) Myocardial Infarction HR (95% CI) Bleeding HR (95% CI) REAL + ZEST LATE 2701 24 vs. ~12 SES, ZES or PES 1. 23 (0. 33 -4. 58) 1. 41 (0. 54 -3. 71) 2. 96 (0. 31 -28. 46) PRODIGY 1357 24 vs 6 PES, ZES, EES 0. 87 (0. 41 -1. 81) 0. 94 (0. 61 -1. 45) 2. 17 (1. 44 -3. 22) EXCELLENT 1443 12 vs 6 SES, EES 0. 17 (0. 02 -1. 39) 0. 54 (0. 21 -1. 35) 2. 0 (0. 37 -11. 11) OPTIMIZE 3120 12 vs 3 ZES 0. 95 (0. 42 -2. 04) 0. 85 (0. 57 -1. 29) 1. 41 (0. 63 -3. 13) ITALIC 1850 12 vs 6 0 vs. 3 events* 0. 67 (0. 19 -2. 38) 3 vs. 0 events* ISAR-SAFE 4005 12 vs 6 0. 80 (0. 21 -3. 03) 1. 08 (0. 51 -2. 27) 1. 25 (0. 34 -4. 76) DAPT 9961 30 vs 12 EES, SES, ZES, BES EES, ZES, SES, PES 0. 29 (0. 17 -0. 48) 0. 47 (0. 37– 0. 61) 1. 61 (1. 21 -2. 16) Type II Error (underpowered studies): Absence of evidence does not constitute evidence of absence Valgimigli, et al. Circulation 2012; 125: 2015. Gwon, et al. Circulation 2012; 125: 505. Park, et al. N Eng J Med 2010; 362: 15. Feres, et al. JAMA 2013; 310: 510. Gillard, et al. J Am Coll Card Nov 2104. Schultz-Schupke, et al. EHJ Jan 25, 2015. 12

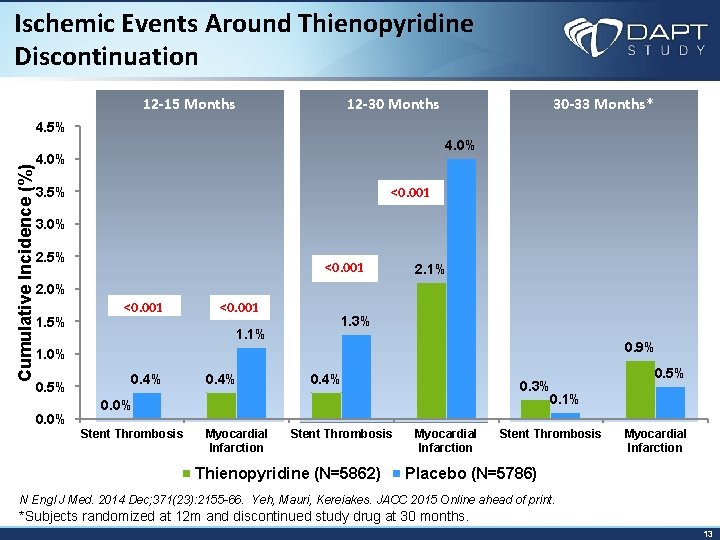
Ischemic Events Around Thienopyridine Discontinuation 12 -15 Months 12 -30 Months 30 -33 Months* Cumulative Incidence (%) 4. 5% 4. 0% <0. 001 3. 5% 3. 0% 2. 5% <0. 001 2. 1% 2. 0% 1. 5% <0. 001 1. 1% 1. 3% 0. 9% 1. 0% 0. 5% 0. 0% 0. 4% 0. 3% 0. 1% 0. 0% Stent Thrombosis Myocardial Infarction Stent Thrombosis Thienopyridine (N=5862) Myocardial Infarction Stent Thrombosis 0. 5% Myocardial Infarction Placebo (N=5786) N Engl J Med. 2014 Dec; 371(23): 2155 -66. Yeh, Mauri, Kereiakes. JACC 2015 Online ahead of print. *Subjects randomized at 12 m and discontinued study drug at 30 months. 13

• Knowing there is a benefit to >12 M, short duration studies of similar power would be expected to be positive so it is not reasonable to randomize the “DAPT” population to shorter duration • However, some patients will need to stop earlier than 1 y (e. g. ineligible for DAPT enrollment or randomization due to bleed, bleed risk, surgery) • Trials of short duration could be designed to • Identify subjects with bleeding>ischemia risk • Quantify time dependent risk of stent thrombosis with interruption/discontinuation in DES with low absolute thrombosis risks 14

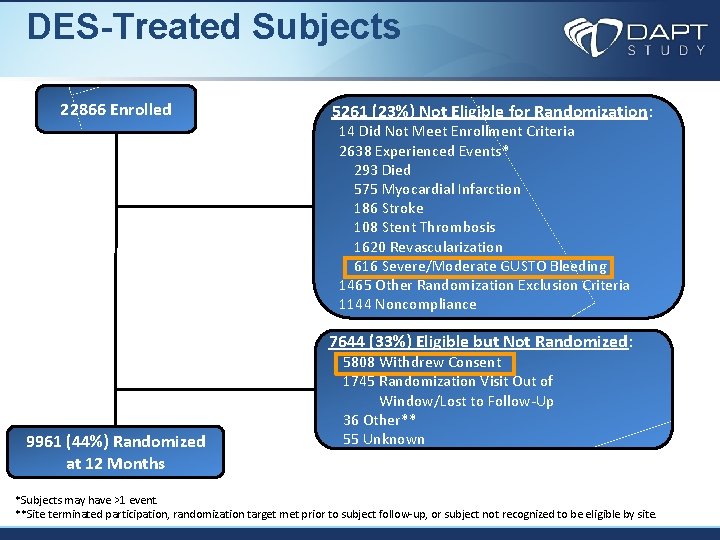
DES-Treated Subjects 22866 Enrolled 5261 (23%) Not Eligible for Randomization: 14 Did Not Meet Enrollment Criteria 2638 Experienced Events* 293 Died 575 Myocardial Infarction 186 Stroke 108 Stent Thrombosis 1620 Revascularization 616 Severe/Moderate GUSTO Bleeding 1465 Other Randomization Exclusion Criteria 1144 Noncompliance 7644 (33%) Eligible but Not Randomized: 9961 (44%) Randomized at 12 Months 5808 Withdrew Consent 1745 Randomization Visit Out of Window/Lost to Follow-Up 36 Other** 55 Unknown *Subjects may have >1 event. **Site terminated participation, randomization target met prior to subject follow-up, or subject not recognized to be eligible by site.

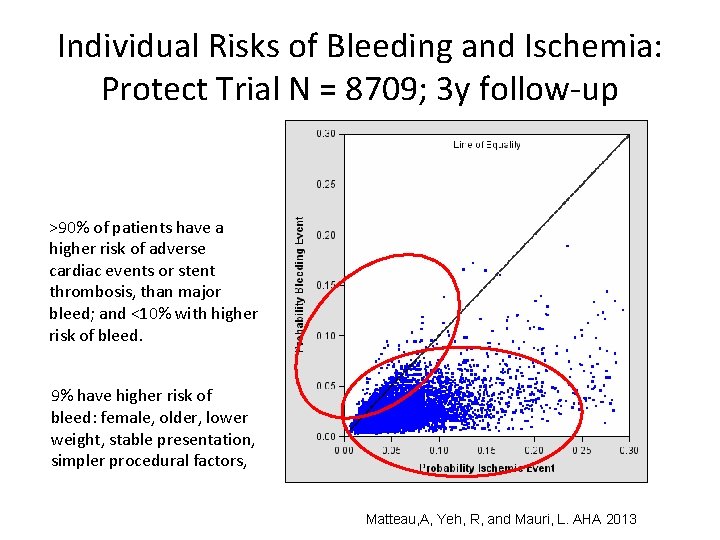
Individual Risks of Bleeding and Ischemia: Protect Trial N = 8709; 3 y follow-up >90% of patients have a higher risk of adverse cardiac events or stent thrombosis, than major bleed; and <10% with higher risk of bleed. 9% have higher risk of bleed: female, older, lower weight, stable presentation, simpler procedural factors, Matteau, A, Yeh, R, and Mauri, L. AHA 2013