DSMB Safety Effectiveness and Blinded versus Unblinded Data
DSMB Safety, Effectiveness, and Blinded versus Unblinded Data Donald E. Cutlip, MD Beth Israel Deaconess Medical Center Baim Institute for Clinical Research Professor of Medicine, Harvard Medical School

Donald Cutlip, MD Salary support: Baim Institute Scientific advisory board: Celonova Corvia

Data and Safety Monitoring Board Food and Drug Administration Guidance Document Guidance for Clinical Trial Sponsors Establishment and Operation of Clinical Trial Data Monitoring Committees https: //www. fda. gov/media/75398/download
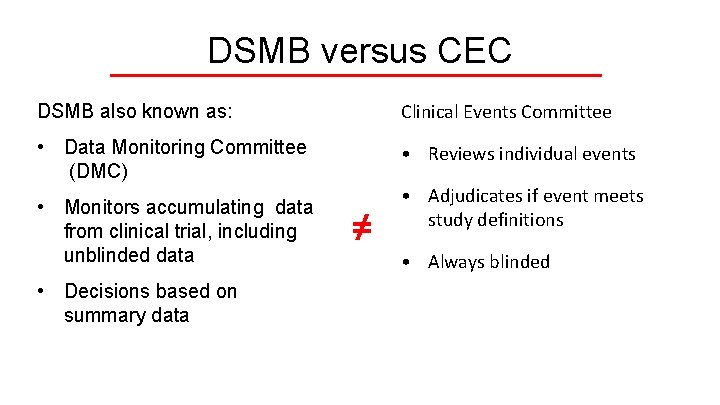
DSMB versus CEC DSMB also known as: Clinical Events Committee • Data Monitoring Committee (DMC) • Reviews individual events • Monitors accumulating data from clinical trial, including unblinded data • Decisions based on summary data ≠ • Adjudicates if event meets study definitions • Always blinded
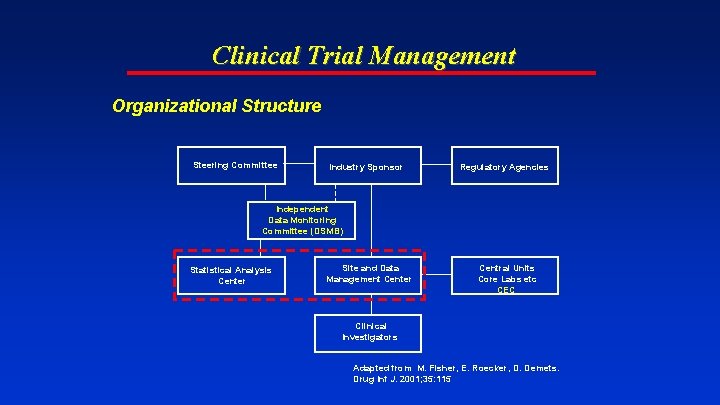
Clinical Trial Management Organizational Structure Steering Committee Industry Sponsor Regulatory Agencies Independent Data Monitoring Committee (DSMB) Statistical Analysis Center Site and Data Management Center Central Units Core Labs etc CEC Clinical Investigators Adapted from M. Fisher, E. Roecker, D. Demets. Drug Inf J. 2001; 35: 115

When is DSMB Needed • All studies are required to perform ongoing monitoring for subject safety. • A formal DSMB is recommended when: – Increased risk to trial participants • Mortality or major morbidity endpoints • Novel therapy or known prior safety concerns • Large, long duration, multi-center trials – higher risk exposure – Issues of possible scientific validity
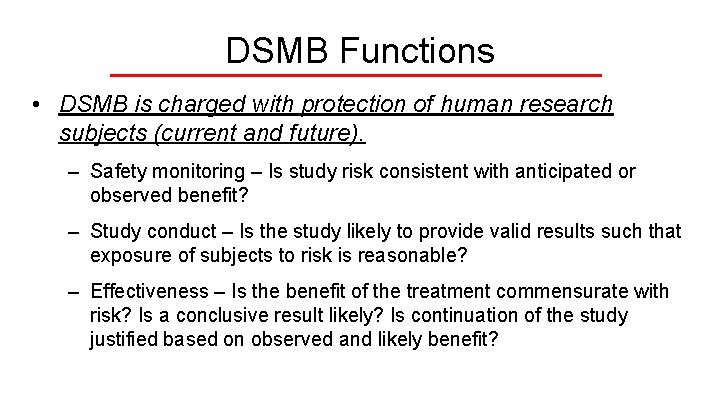
DSMB Functions • DSMB is charged with protection of human research subjects (current and future). – Safety monitoring – Is study risk consistent with anticipated or observed benefit? – Study conduct – Is the study likely to provide valid results such that exposure of subjects to risk is reasonable? – Effectiveness – Is the benefit of the treatment commensurate with risk? Is a conclusive result likely? Is continuation of the study justified based on observed and likely benefit?

DSMB Members • Chair – Clinician or statistician experienced in DSMB procedures and responsibilities • Clinicians – 2 -4 clinical experts in therapeutic area of the study. At least 1 clinician should have prior DSMB experience • Statistician – experience in DSMB statistical methods especially related to stopping rules and guidance for early stopping due to safety or futility. • Other members – depending on the study other members may be necessary (eg. ethicist, patient representative)

DSMB Responsibilities • Maintain and confirm independence from study sponsor, investigators, and other trial participants • Maintain confidentiality regarding data reviewed • Review accumulating data on periodic basis as specified in the DSMB charter or as needed based on trial performance and concerns. • Make judgement regarding study continuing with or without modifications • Communicate findings and recommendations to sponsor or designee (Executive/Steering Committee) in timely manner.

DSMB Safety Review • Safety endpoints (eg. MI, stent thrombosis, stroke); preferably adjudicated by CEC • Site reported adverse events; preferably coded by system class to allow assessment for trends • Treatment (drug/device) related adverse events • Usually not formal stopping rules for safety analysis; DSMB may modify or recommend stopping trial based on perceived safety concerns; statistical significance not usually required or wise
DSMB Effectiveness Review • Effectiveness endpoints - preferably adjudicated by CEC • Surrogate endpoints that may reflect early effectiveness • DSMB may be charged with reviewing interim analysis for overwhelming efficacy or futility; sample size adjustment (adaptive design) – Alpha penalties for interim looks – Usually require very strong significance for stopping early (certainty of conclusions; preserve alpha for study final analysis as designed) – Futility analyses avoid risks to added subjects if conclusive result unlikely. – Sample size adjustment based on conditional power – “adaptive design”.

DSMB Study Conduct Review • Enrollment trends – Planned vs. observed enrollment rates – Enrollment per site • Protocol deviations – Impact on trial validity and data integrity • Data Compliance – Data submission and follow-up – Reporting of AEs – Endpoint assessments
DSMB – Blinded or Unblinded • Little if any cause for DSMB to be blinded • Purpose of blinding in clinical trials – Limit bias in results – Avoid premature release of results • Difficult for DSMB to meet responsibilities if blinded – Blinded net results may miss important safety signals
- Slides: 13