DSM TACE in comparison with c and DEBTACE
DSM – TACE in comparison with c- and DEB-TACE Embo. Cept® S, DSM 35 – 50* *) DSM = degradable starch microspheres (Amilomer) 1
DSM (EMBOCEPT® S) IN COMPARISON Embo. Cept® S vs. DC Beads § Pharmacokinetics § Clinics § Therapeutic/technical recommendation vs. lipiodol 2
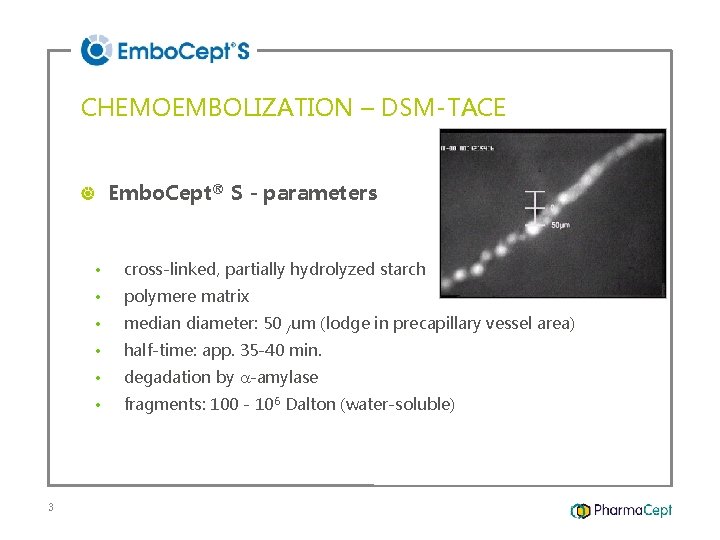
CHEMOEMBOLIZATION – DSM-TACE Embo. Cept® S - parameters 3 • cross-linked, partially hydrolyzed starch • polymere matrix • median diameter: 50 /um (lodge in precapillary vessel area) • half-time: app. 35 -40 min. • degadation by -amylase • fragments: 100 - 106 Dalton (water-soluble)
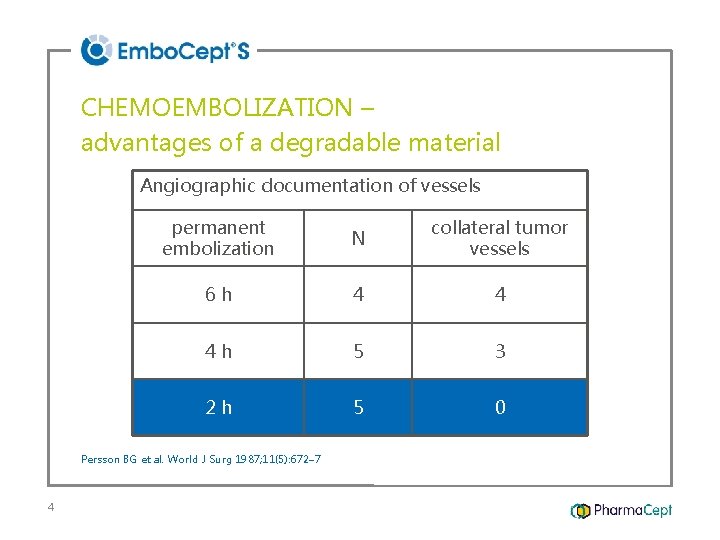
CHEMOEMBOLIZATION – advantages of a degradable material Angiographic documentation of vessels permanent embolization N collateral tumor vessels 6 h 4 4 4 h 5 3 2 h 5 0 Persson BG et al. World J Surg 1987; 11(5): 672– 7 4
DSM (EMBOCEPT® S) VS. DC BEADS pharmacokinetics clinics therapeutic/technical recommendation 5
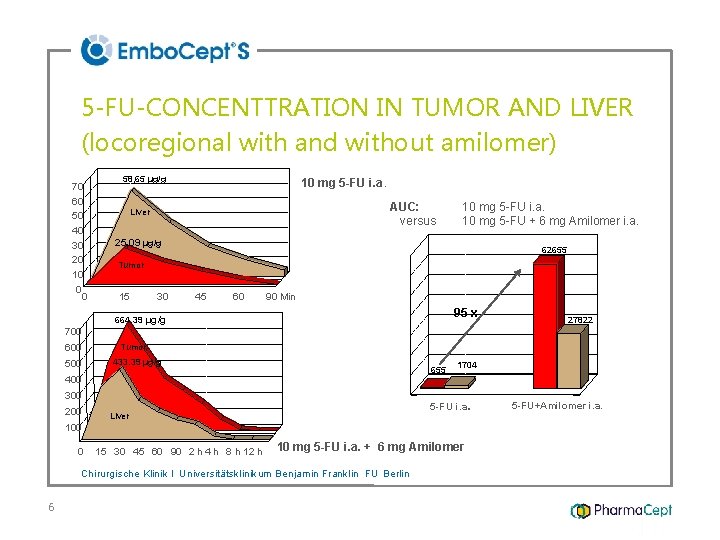
70 60 50 40 30 20 10 0 0 58, 65 µg/g AUC: versus Liver 25, 09 µg/g Tumor 15 30 664. 39 µg/g 700 600 µg/g 5 -FU 500 10 mg 5 -FU i. a. Tumor 433. 39 µg/g 45 60 90 Min AUC (15 -120 min) µg/g*min µg/g 5 -FU-CONCENTTRATION IN TUMOR AND LIVER (locoregional with and without amilomer) 60000 200 62655 50000 40000 95 x 10000 0 Liver 655 1704 5 -FU i. a. 100 0 0 10 mg 5 -FU i. a. + 6 mg Amilomer 15 30 45 60 90 2 h 4 h 8 h 12 h 24 h Chirurgische Klinik I Universitätsklinikum Benjamin Franklin FU Berlin 6 27822 20000 400 300 10 mg 5 -FU i. a. 10 mg 5 -FU + 6 mg Amilomer i. a. 5 -FU+Amilomer i. a.

PHARMACOKINETIC WITH DE BEADS: TUMOR DOXORUBICIN CONCENTRATION 450 Doxorubicin in tumor (nmoles/g) 400 350 300 250 HAI 200 DE Beads 150 100 50 0 0 100 200 300 Time from doxorubicin administration (hours) Hong K, et al. Clin Cancer Res 2006; 12: 2563– 7, Courtesy: R. Lencioni, Pisa 7 400
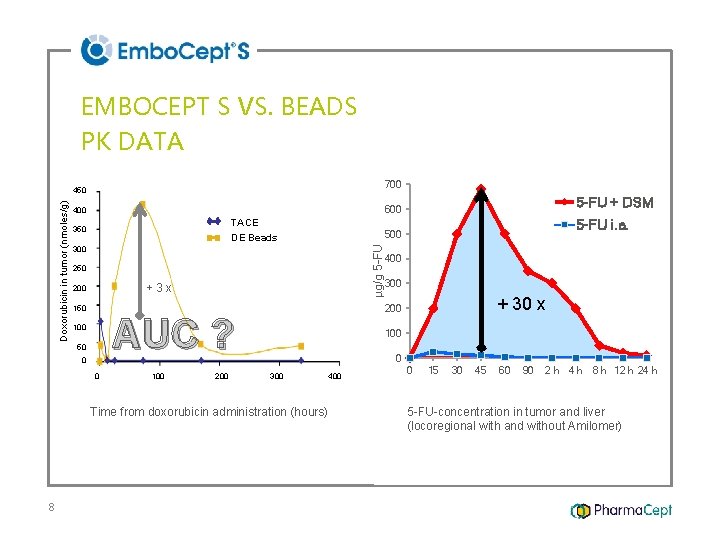
EMBOCEPT S VS. BEADS PK DATA 700 TACE 350 300 250 +3 x 200 150 0 0 100 200 400 300 + 30 x 200 AUC ? 100 5 -FU i. a. 500 DE Beads 50 100 0 300 400 Time from doxorubicin administration (hours) 8 5 -FU + DSM 600 400 µg/g 5 -FU Doxorubicin in tumor (nmoles/g) 450 0 15 30 45 60 90 2 h 4 h 8 h 12 h 24 h 5 -FU-concentration in tumor and liver (locoregional with and without Amilomer)
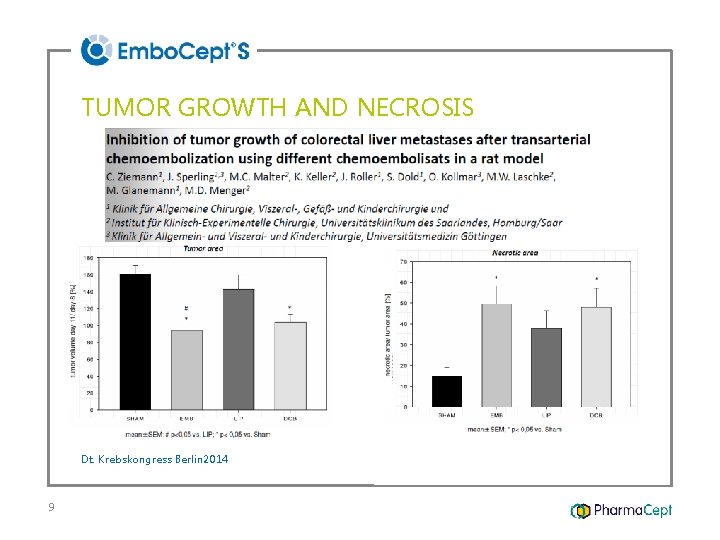
TUMOR GROWTH AND NECROSIS Dt. Krebskongress Berlin 2014 9
DSM (EMBOCEPT® S) VS. DC BEADS pharmacokinetics clinics therapeutic/technical recommendation 10
DSM – CLINICAL DATA clinics (TACE) Taguchi, T. (1992): random. phase III - study indications: Taguchi et al. Reg Cancer Treatment 3 -4: 117 1992 11 1) HCC (n=60) 2) liver metastases (n=60)
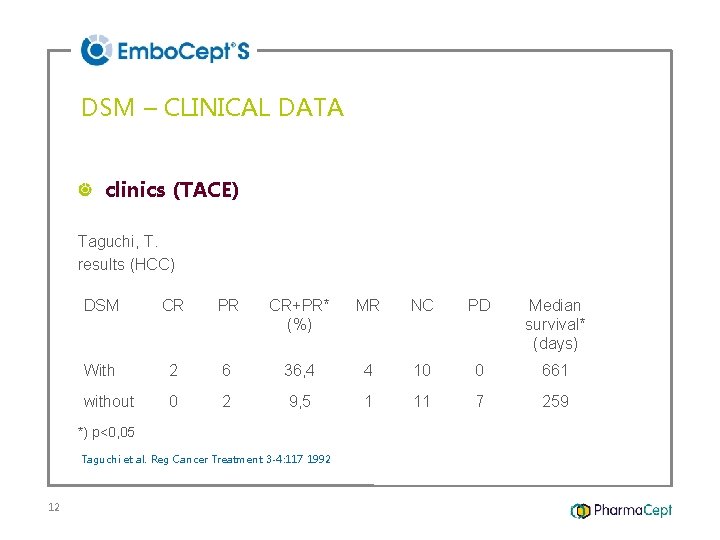
DSM – CLINICAL DATA clinics (TACE) Taguchi, T. results (HCC) DSM CR PR CR+PR* MR (%) NC PD Median survival* (days) With 2 6 36, 4 without 0 2 9, 5 1 *) p<0, 05 Taguchi et al. Reg Cancer Treatment 3 -4: 117 1992 12 4 10 11 0 7 661 259
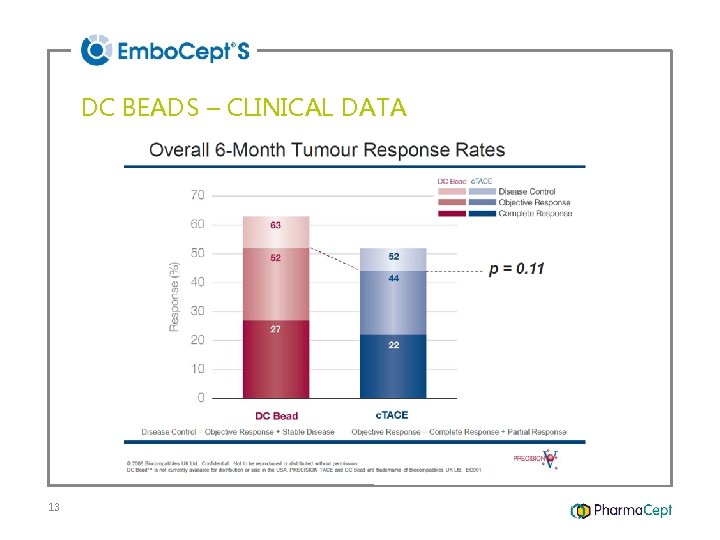
DC BEADS – CLINICAL DATA 13
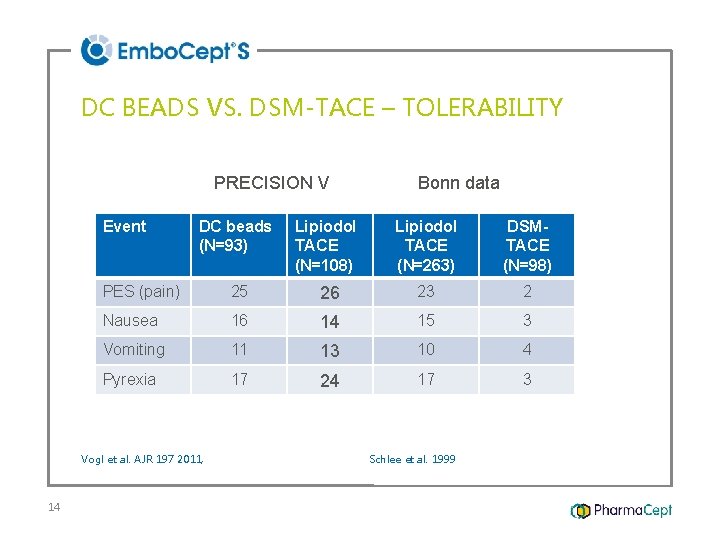
DC BEADS VS. DSM-TACE – TOLERABILITY PRECISION V Event DC beads (N=93) Lipiodol TACE (N=108) Lipiodol TACE (N=263) DSMTACE (N=98) PES (pain) 25 26 23 2 Nausea 16 14 15 3 Vomiting 11 13 10 4 Pyrexia 17 24 17 3 Vogl et al. AJR 197 2011, 14 Bonn data Schlee et al. 1999
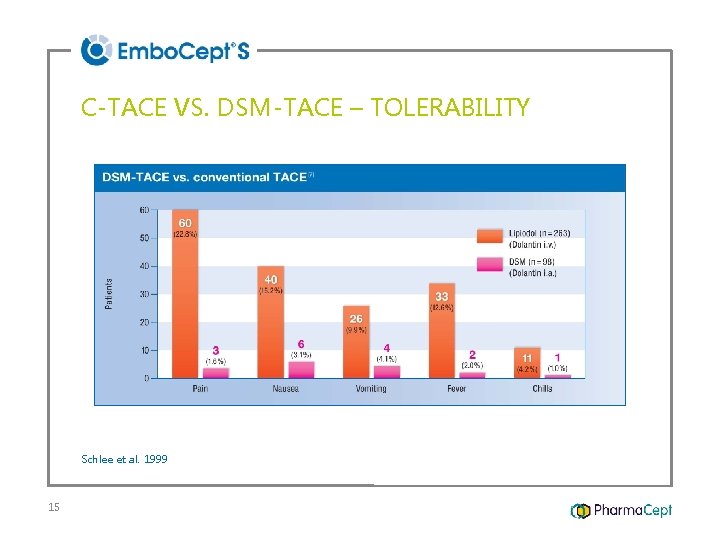
C-TACE VS. DSM-TACE – TOLERABILITY Schlee et al. 1999 15
Sequence of repeated non-selective nonocclusive TACE of far advanced HCC with degradable starch microspheres (DSM) – long term results of a retrospective analysis Thomas Albrecht, Julian Wirsching, Gerd Berger Institut für Radiologie und Interventionelle Therapie Vivantes-Klinikum Neukölln, Berlin Charité, Campus BF, Berlin thomas. albrecht@vivantes. de 16
DSM (EMBOCEPT® S) VS. DC BEADS pharmacokinetics clinics therapeutic/technical recommendation 17
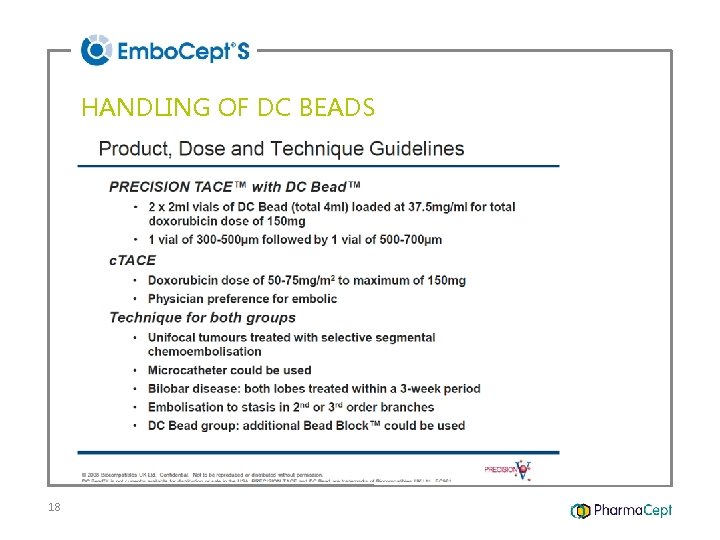
HANDLING OF DC BEADS 18
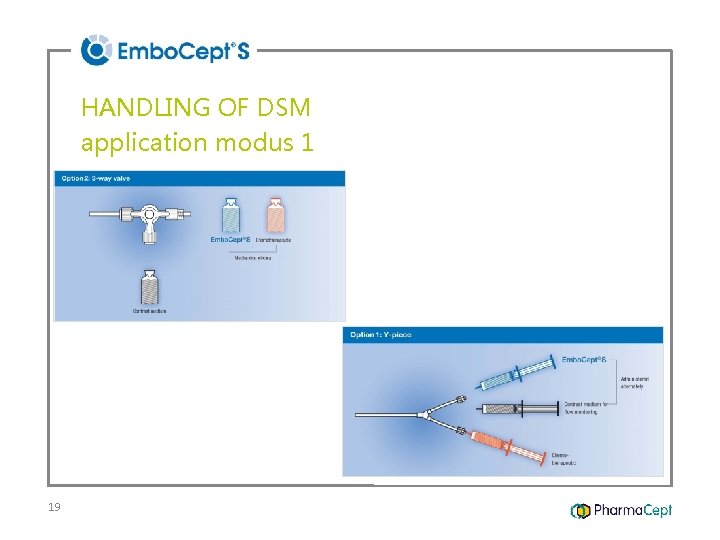
HANDLING OF DSM application modus 1 19
HANDLING OF DSM application modus 2 20
HANDLING OF DSM application modus 3 21
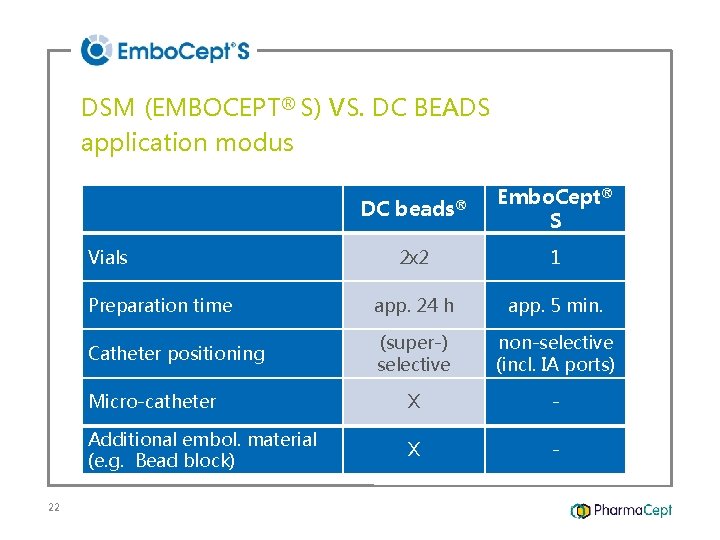
DSM (EMBOCEPT® S) VS. DC BEADS application modus DC beads® Embo. Cept® S 2 x 2 1 Preparation time app. 24 h app. 5 min. Catheter positioning (super-) selective non-selective (incl. IA ports) Micro-catheter X - Additional embol. material (e. g. Bead block) X - Vials 22
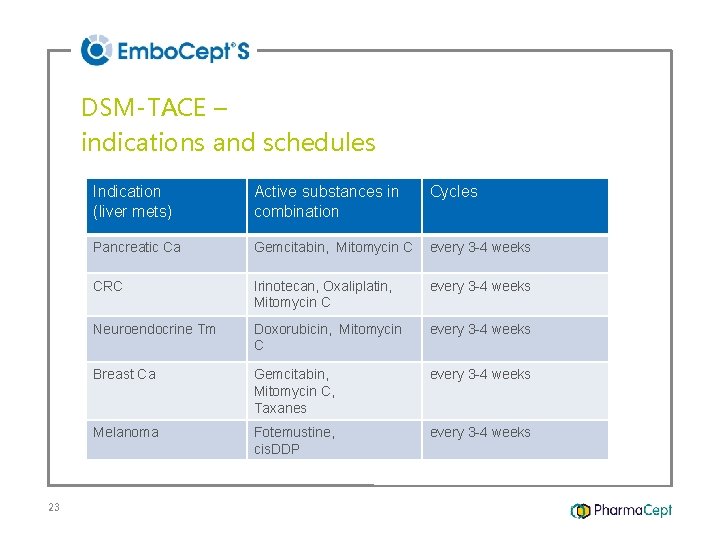
DSM-TACE – indications and schedules 23 Indication (liver mets) Active substances in combination Cycles Pancreatic Ca Gemcitabin, Mitomycin C every 3 -4 weeks CRC Irinotecan, Oxaliplatin, Mitomycin C every 3 -4 weeks Neuroendocrine Tm Doxorubicin, Mitomycin C every 3 -4 weeks Breast Ca Gemcitabin, Mitomycin C, Taxanes every 3 -4 weeks Melanoma Fotemustine, cis. DDP every 3 -4 weeks
DSM (EMBOCEPT® S) IN COMPARISON Embo. Cept® S vs. DC Beads Pharmacokinetics Clinics Therapeutic/technical recommendation vs. lipiodol 24
CHEMOEMBOLIZATION – LIPIODOL ® + / - DSM 25
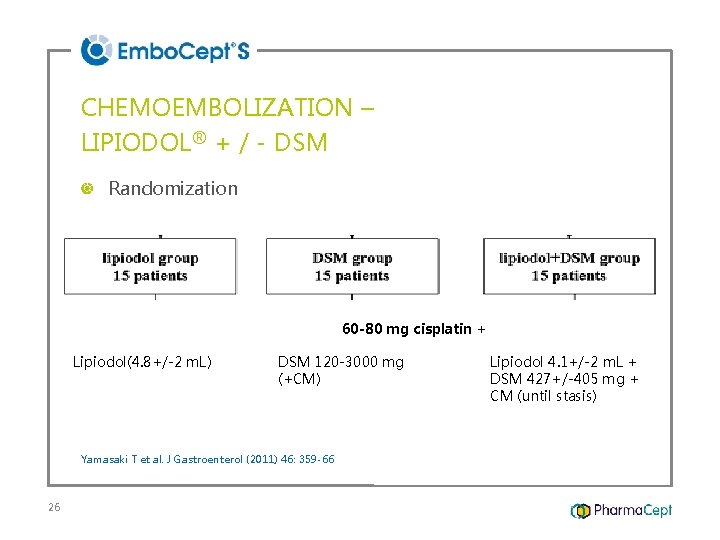
CHEMOEMBOLIZATION – LIPIODOL® + / - DSM Randomization 60 -80 mg cisplatin + Lipiodol(4. 8+/-2 m. L) DSM 120 -3000 mg (+CM) Yamasaki T et al. J Gastroenterol (2011) 46: 359 -66 26 Lipiodol 4. 1+/-2 m. L + DSM 427+/-405 mg + CM (until stasis)
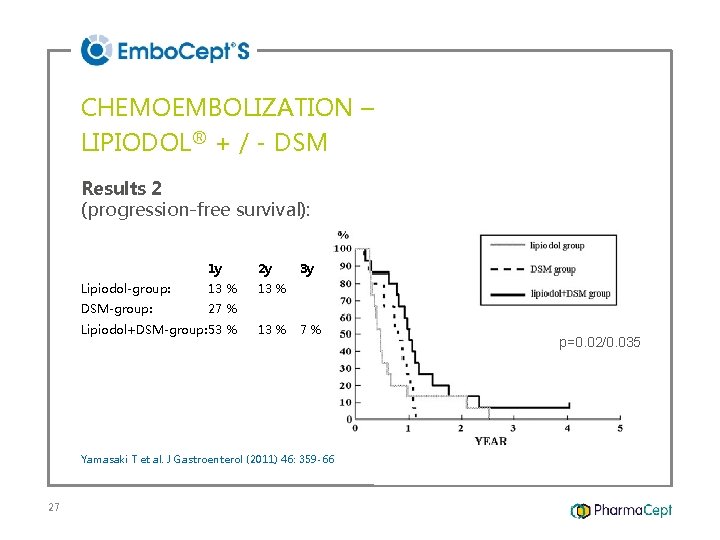
CHEMOEMBOLIZATION – LIPIODOL® + / - DSM Results 2 (progression-free survival): 1 y 2 y Lipiodol-group: 13 % DSM-group: 27 % Lipiodol+DSM-group: 53 % 13 % 3 y 7% Yamasaki T et al. J Gastroenterol (2011) 46: 359 -66 27 p=0. 02/0. 035
TRANSARTERIAL CHEMOEMBOLIZATION (TACE) of hepatocellular carcinoma (HCC): Comparison of tumor response rates between c(lipiodol)-TACE and DSM-TACE plus lipiodol Gruber-Rouh T, Nagy N, Eichler K, Lehnert T, Harth M, Zangos S, Beeres M, Nour-Eldin NE, Vogl TJ Institut of Diagnostic and Interventional Radiology Johann Wolfgang Goethe-University, Frankfurt am Main 28
PURPOSE Evaluation and comparison of local tumor response under transarterial chemoembolization (TACE) of HCC with lipiodol or lipiodol plus DSM-microspheres (Embo. Cept® S) Institut of Diagnostic and Interventional Radiology Johann Wolfgang Goethe-University, Frankfurt am Main 29
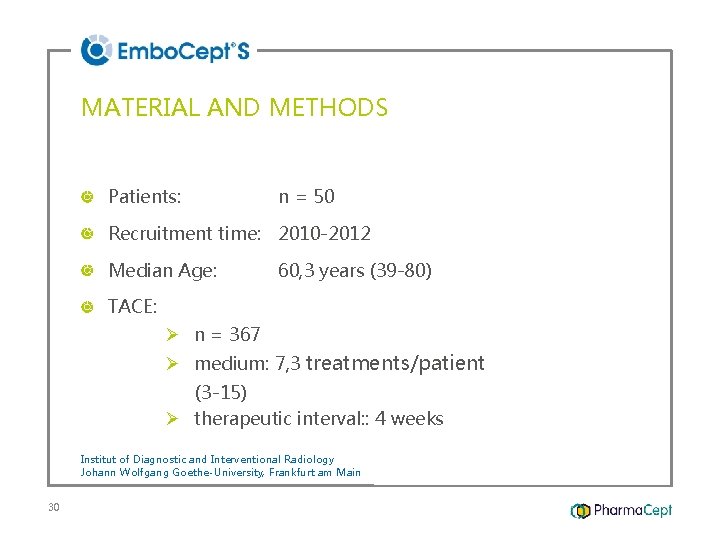
MATERIAL AND METHODS Patients: n = 50 Recruitment time: 2010 -2012 Median Age: 60, 3 years (39 -80) TACE: Ø n = 367 Ø medium: 7, 3 treatments/patient (3 -15) Ø therapeutic interval: : 4 weeks Institut of Diagnostic and Interventional Radiology Johann Wolfgang Goethe-University, Frankfurt am Main 30
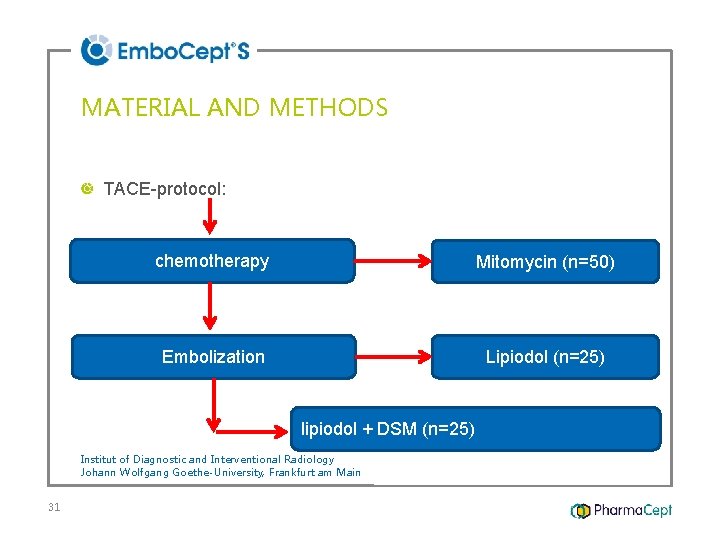
MATERIAL AND METHODS TACE-protocol: chemotherapy Mitomycin (n=50) Embolization Lipiodol (n=25) lipiodol + DSM (n=25) Institut of Diagnostic and Interventional Radiology Johann Wolfgang Goethe-University, Frankfurt am Main 31
MATERIAL AND METHODS Evaluation: Radilogical tumor response: MRT, CT (RECIST-criterias) Statistical significance: (Wilcoxon-Mann-Whitney-Test) Institut of Diagnostic and Interventional Radiology Johann Wolfgang Goethe-University, Frankfurt am Main 32
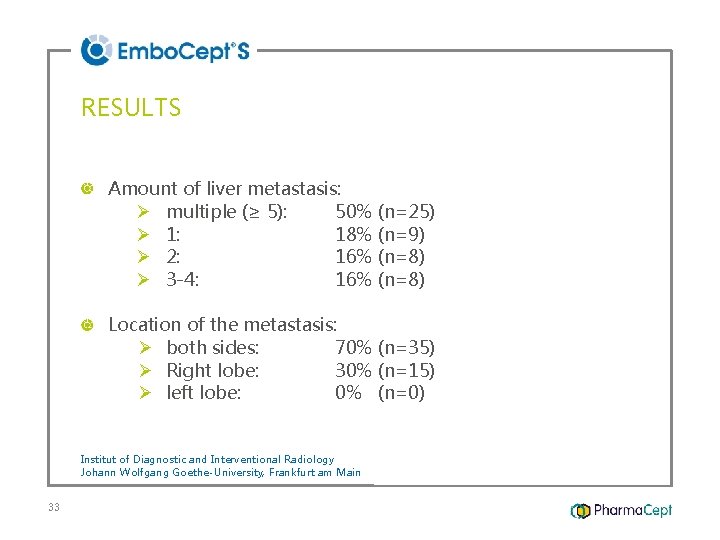
RESULTS Amount of liver metastasis: Ø multiple (≥ 5): 50% (n=25) Ø 1: 18% (n=9) Ø 2: 16% (n=8) Ø 3 -4: 16% (n=8) Location of the metastasis: Ø both sides: 70% (n=35) Ø Right lobe: 30% (n=15) Ø left lobe: 0% (n=0) Institut of Diagnostic and Interventional Radiology Johann Wolfgang Goethe-University, Frankfurt am Main 33
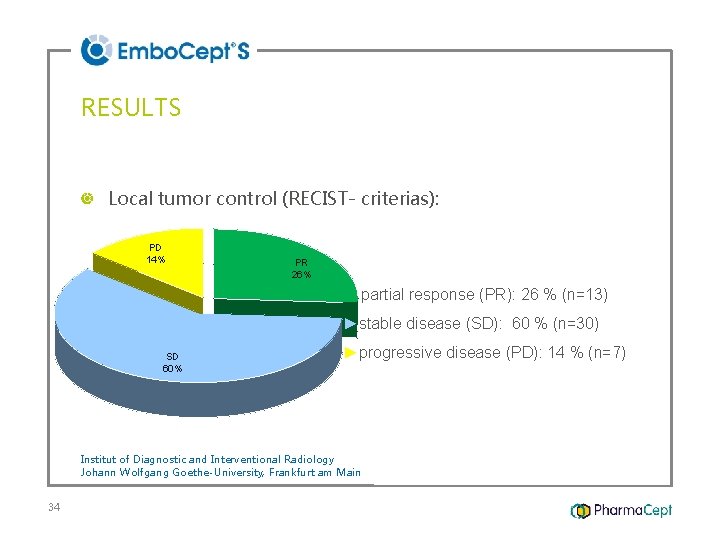
RESULTS Local tumor control (RECIST- criterias): PD 14% PR 26% ►partial response (PR): 26 % (n=13) ►stable disease (SD): 60 % (n=30) SD 60% ►progressive disease (PD): 14 % (n=7) Institut of Diagnostic and Interventional Radiology Johann Wolfgang Goethe-University, Frankfurt am Main 34
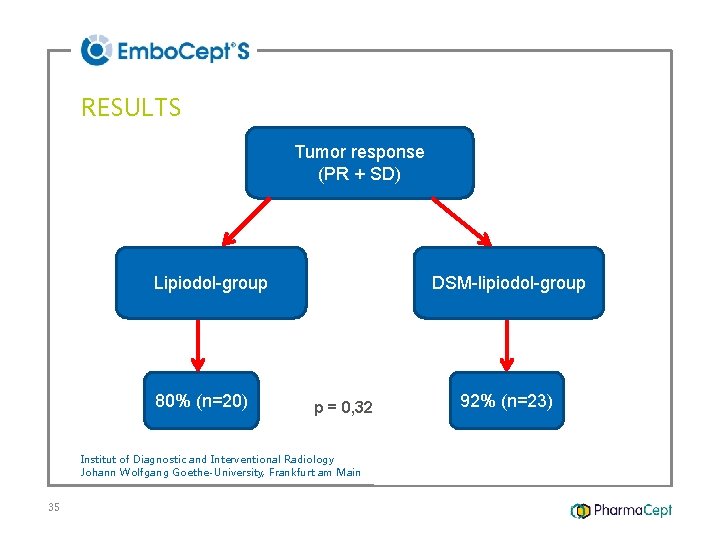
RESULTS Tumor response (PR + SD) Lipiodol-group 80% (n=20) DSM-lipiodol-group p = 0, 32 Institut of Diagnostic and Interventional Radiology Johann Wolfgang Goethe-University, Frankfurt am Main 35 92% (n=23)
SUMMARY 1/4 DSM – TACE: highest accumulation of co-applicated drugs within the tumor tissue Embo. Cept® S (DSM 35/50) – short-time embolization: • • 36 no initiation signals for collateral tumor vessel comparable high tumor necrosis
SUMMARY 2/4 DSM-TACE comparable to DEB-TACE in tumor response, but better in tolerability DSM-TACE better than c-TACE 37
SUMMARY 3/4 DSM-TACE: options for new scientific concepts combination with: • LITT/RF, • Radiation / SIRT • immune-/ genembolization 38
SUMMARY 4/4 Embo. Cept® S: unique chemoembolization material 39 • combination • indication • application
- Slides: 39