Dry Reforming using with Plasma Plasma Application for




![CH 4 conversion – different plasma generation CH 4 conv. [%] SED [KJ/L] Type CH 4 conversion – different plasma generation CH 4 conv. [%] SED [KJ/L] Type](https://slidetodoc.com/presentation_image_h/a1abe442343be2048730c0caa5586692/image-32.jpg)
- Slides: 36

Dry Reforming using with Plasma “Plasma Application for Energy & Environment” Korea Institute of Machinery & Materials (KIMM) Young-Hoon Song 2013. 08

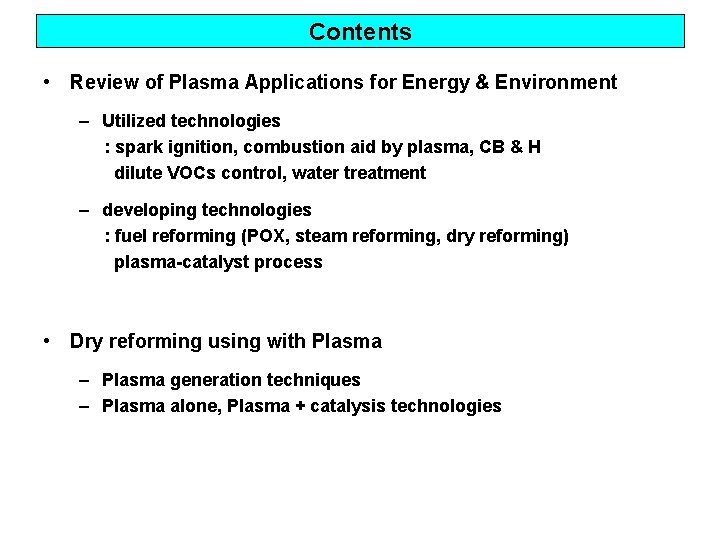
Contents • Review of Plasma Applications for Energy & Environment – Utilized technologies : spark ignition, combustion aid by plasma, CB & H dilute VOCs control, water treatment – developing technologies : fuel reforming (POX, steam reforming, dry reforming) plasma-catalyst process • Dry reforming using with Plasma – Plasma generation techniques – Plasma alone, Plasma + catalysis technologies

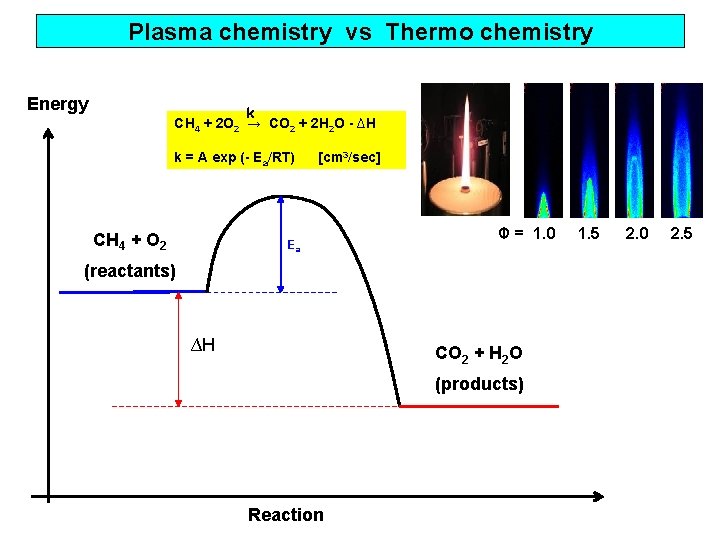
Plasma chemistry vs Thermo chemistry Energy k CH 4 + 2 O 2 → CO 2 + 2 H 2 O - ∆H k = A exp (- Ea/RT) CH 4 + O 2 [cm 3/sec] Ea Φ = 1. 0 (reactants) ∆H CO 2 + H 2 O (products) Reaction 1. 5 2. 0 2. 5

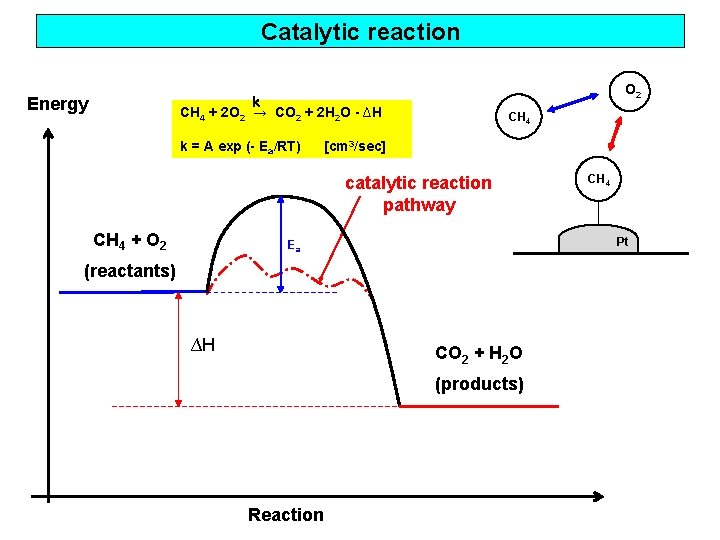
Catalytic reaction Energy O 2 k CH 4 + 2 O 2 → CO 2 + 2 H 2 O - ∆H k = A exp (- Ea/RT) CH 4 [cm 3/sec] catalytic reaction pathway CH 4 + O 2 Pt Ea (reactants) ∆H CO 2 + H 2 O (products) Reaction CH 4

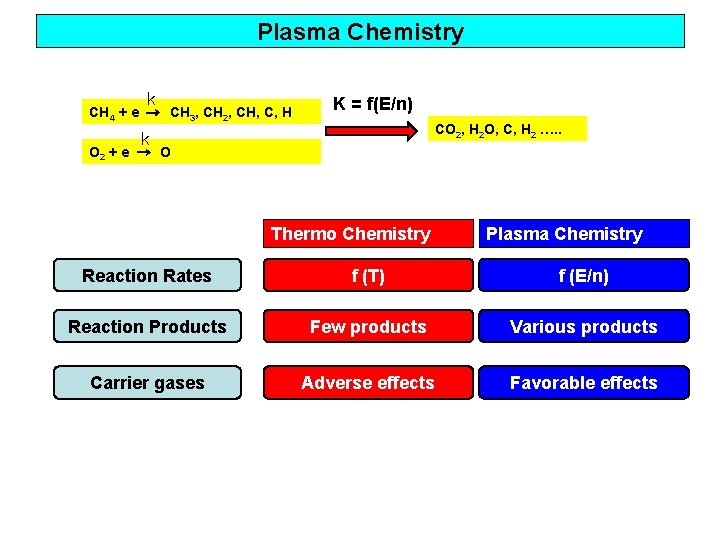
Plasma Chemistry k CH 4 + e → CH 3, CH 2, CH, C, H K = f(E/n) CO 2, H 2 O, C, H 2 …. . k O 2 + e → O Thermo Chemistry Plasma Chemistry Reaction Rates f (T) f (E/n) Reaction Products Few products Various products Carrier gases Adverse effects Favorable effects

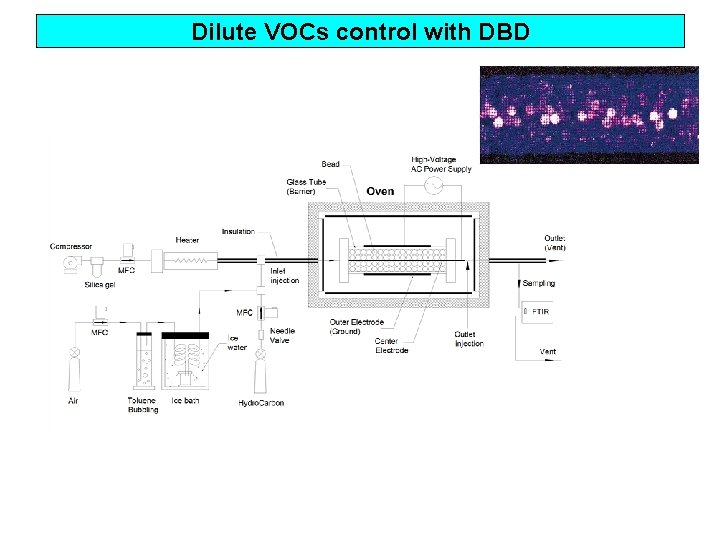
Dilute VOCs control with DBD

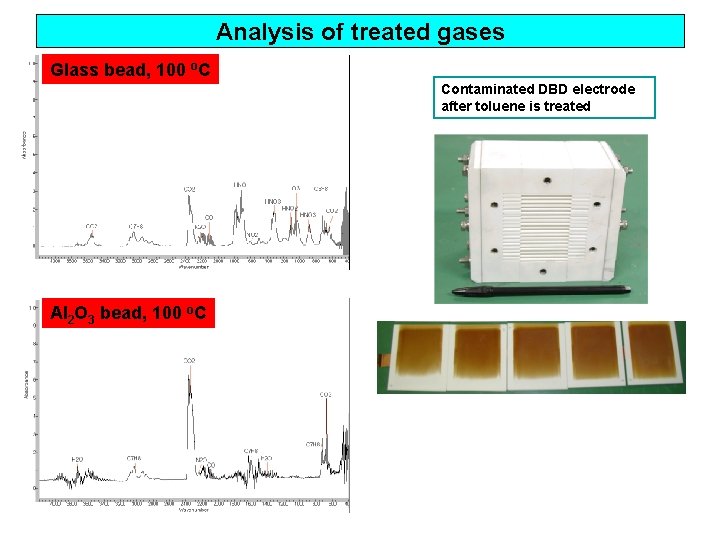
Analysis of treated gases Glass bead, 100 o. C Contaminated DBD electrode after toluene is treated Al 2 O 3 bead, 100 o. C

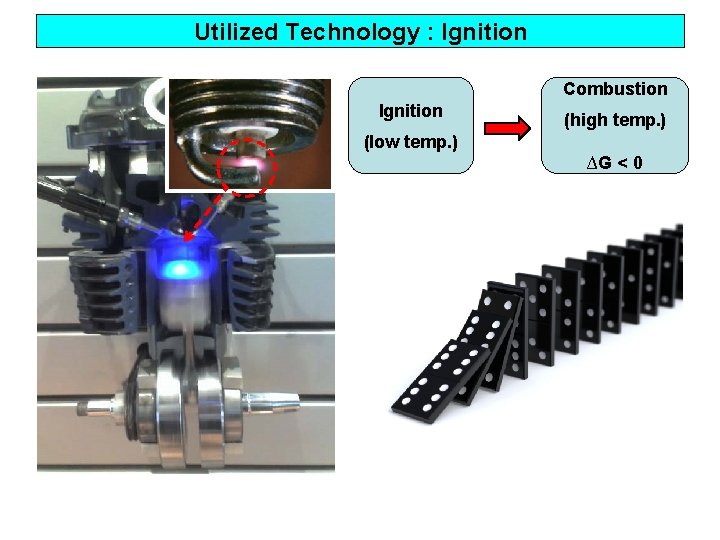
Utilized Technology : Ignition Combustion Ignition (high temp. ) (low temp. ) ∆G < 0

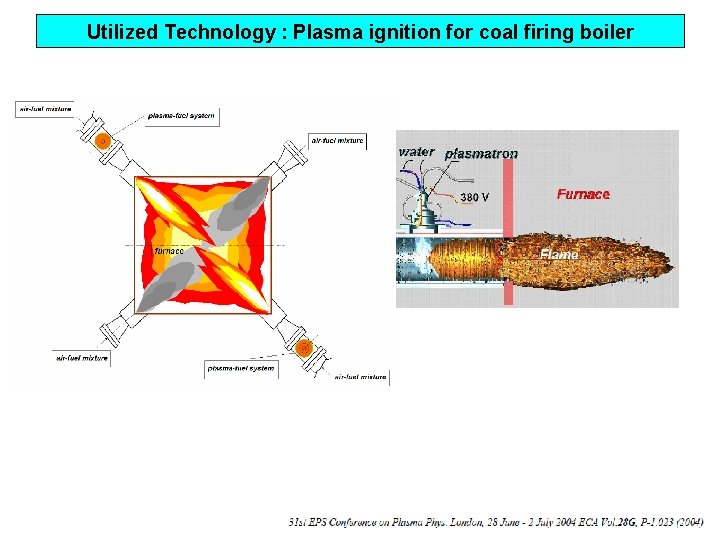
Utilized Technology : Plasma ignition for coal firing boiler

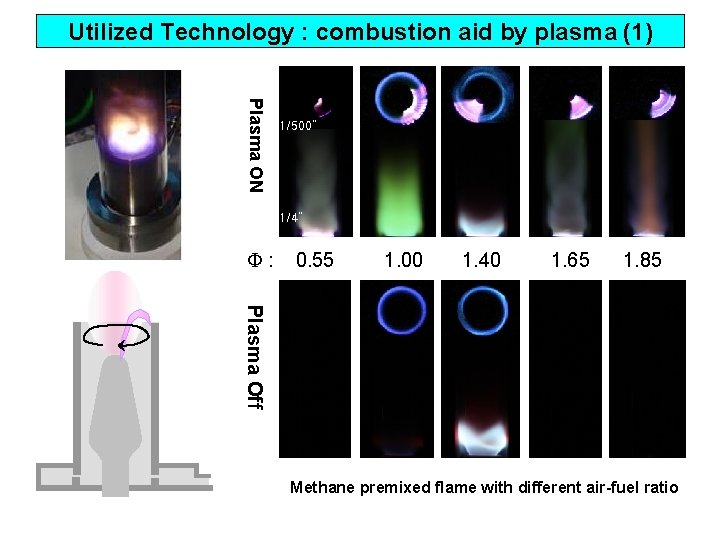
Utilized Technology : combustion aid by plasma (1) Plasma ON 1/500” 1/4” : 0. 55 1. 00 1. 40 1. 65 1. 85 Plasma Off Methane premixed flame with different air-fuel ratio

Utilized Technology : combustion aid by plasma (2) Plasma burner for diesel after-treatment

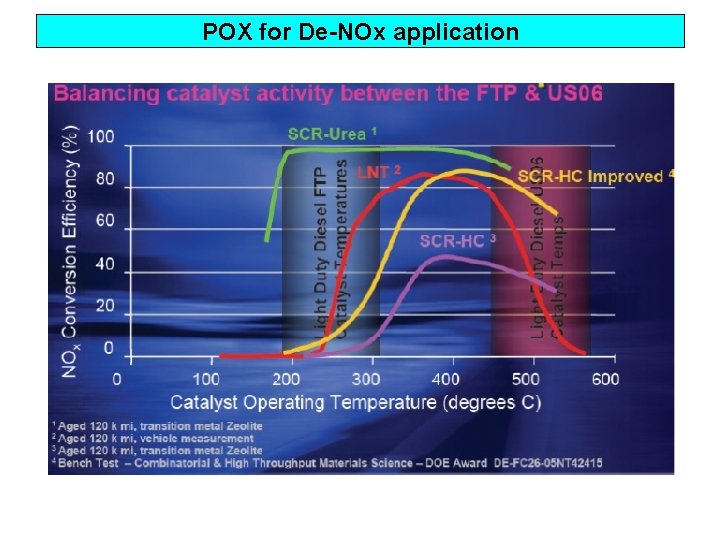
POX for De-NOx application

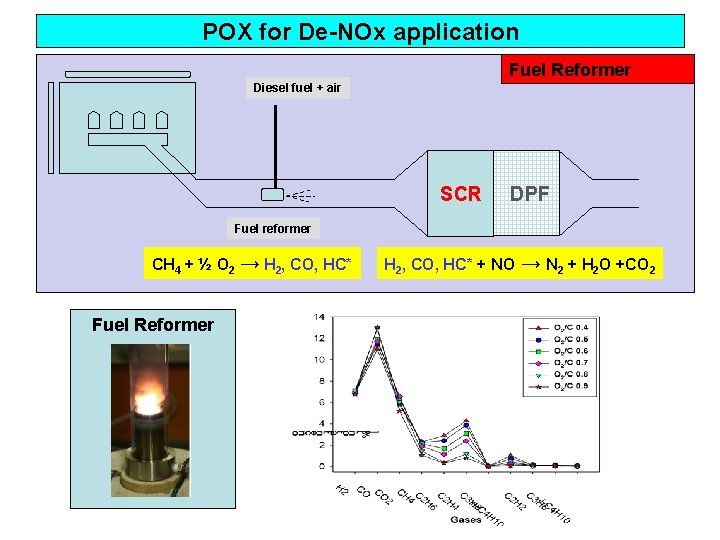
POX for De-NOx application Fuel Reformer Diesel fuel + air SCR DPF Fuel reformer CH 4 + ½ O 2 → H 2, CO, HC* Fuel Reformer H 2, CO, HC* + NO → N 2 + H 2 O +CO 2

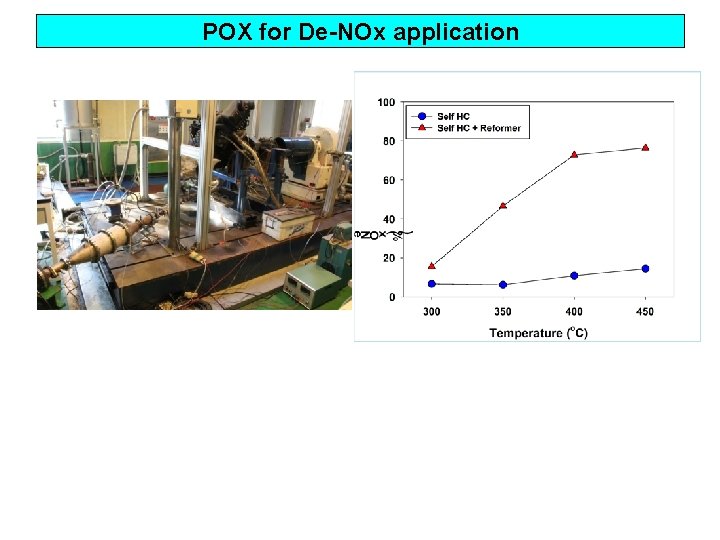
POX for De-NOx application

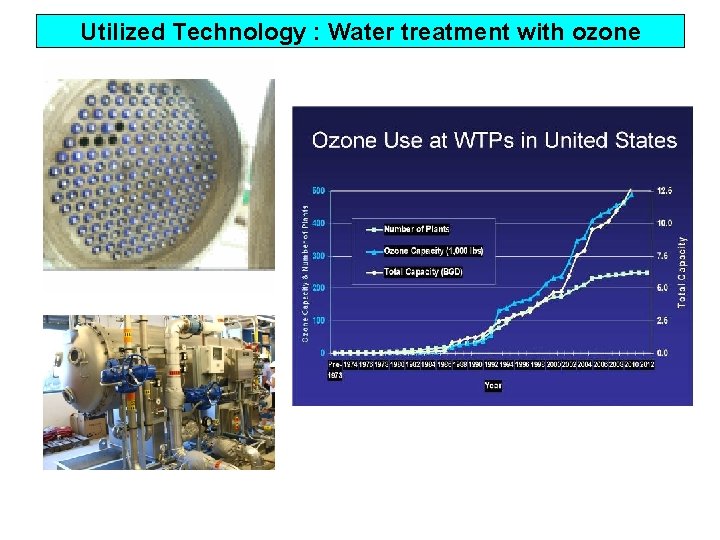
Utilized Technology : Water treatment with ozone

Utilized Technology : Dilute VOCs & Odor Control Takuma Co. Plasma Deodorization

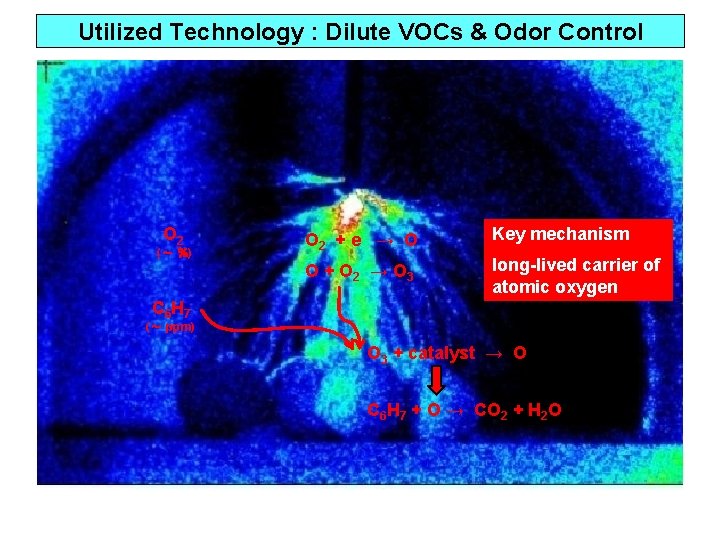
Utilized Technology : Dilute VOCs & Odor Control O 2 (∼ %) O 2 + e → O Key mechanism O + O 2 → O 3 long-lived carrier of atomic oxygen C 6 H 7 (∼ ppm) O 3 + catalyst → O C 6 H 7 + O → CO 2 + H 2 O


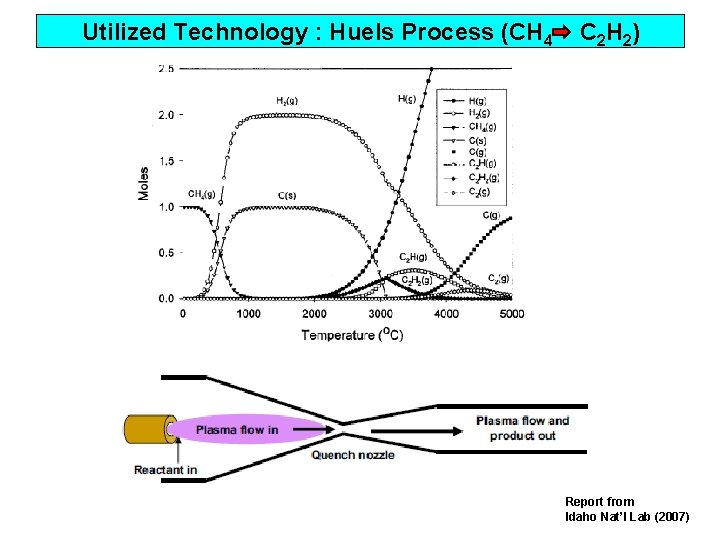
Utilized Technology : Huels Process (CH 4 C 2 H 2) Report from Idaho Nat’l Lab (2007)

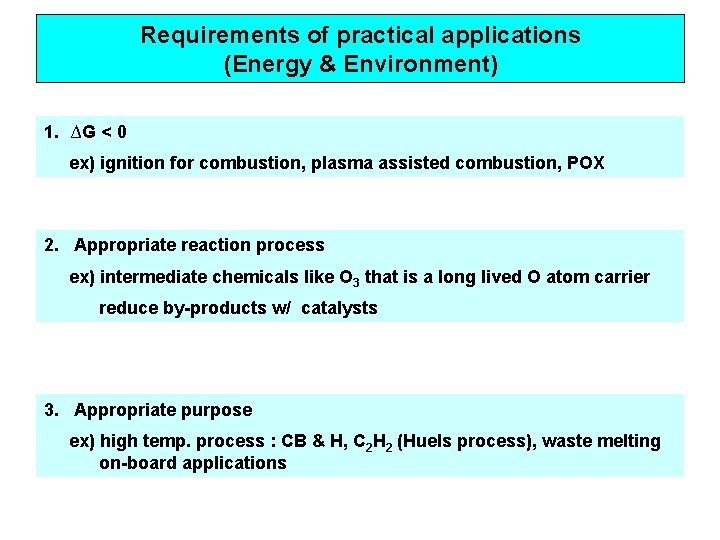
Requirements of practical applications (Energy & Environment) 1. ∆G < 0 ex) ignition for combustion, plasma assisted combustion, POX 2. Appropriate reaction process ex) intermediate chemicals like O 3 that is a long lived O atom carrier reduce by-products w/ catalysts 3. Appropriate purpose ex) high temp. process : CB & H, C 2 H 2 (Huels process), waste melting on-board applications

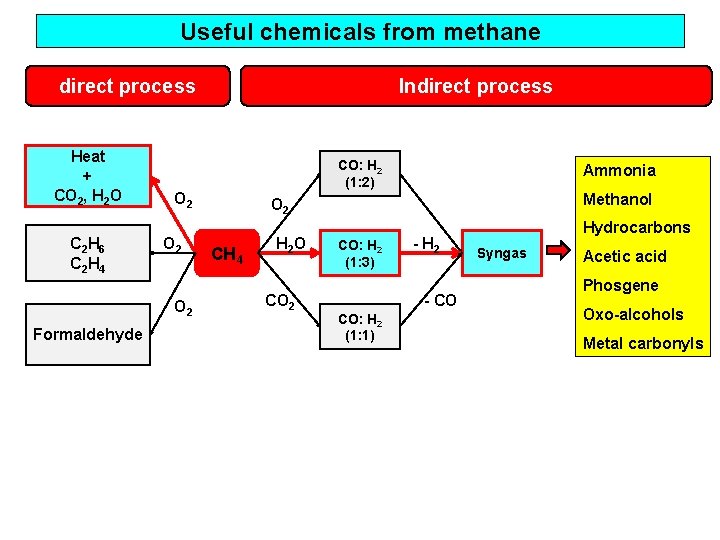
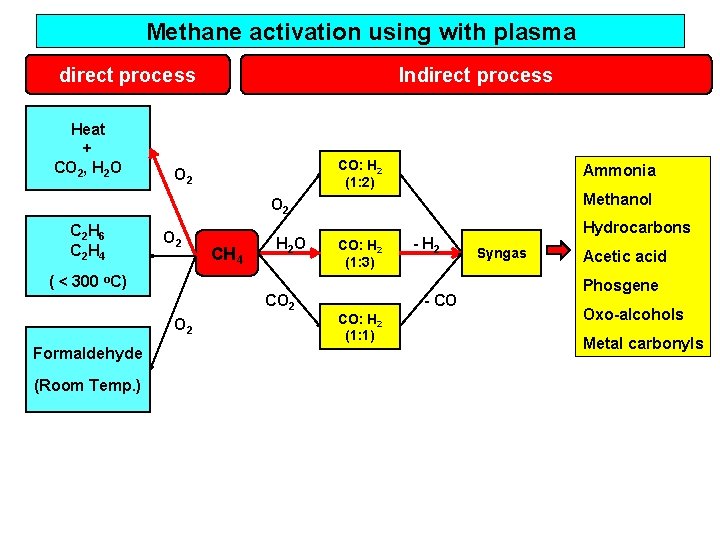
Useful chemicals from methane direct process Heat + CO 2, H 2 O C 2 H 6 C 2 H 4 CO: H 2 (1: 2) O 2 O 2 Formaldehyde Indirect process Ammonia Methanol O 2 CH 4 H 2 O CO 2 CO: H 2 (1: 3) - H 2 - CO CO: H 2 (1: 1) Hydrocarbons Syngas Acetic acid Phosgene Oxo-alcohols Metal carbonyls

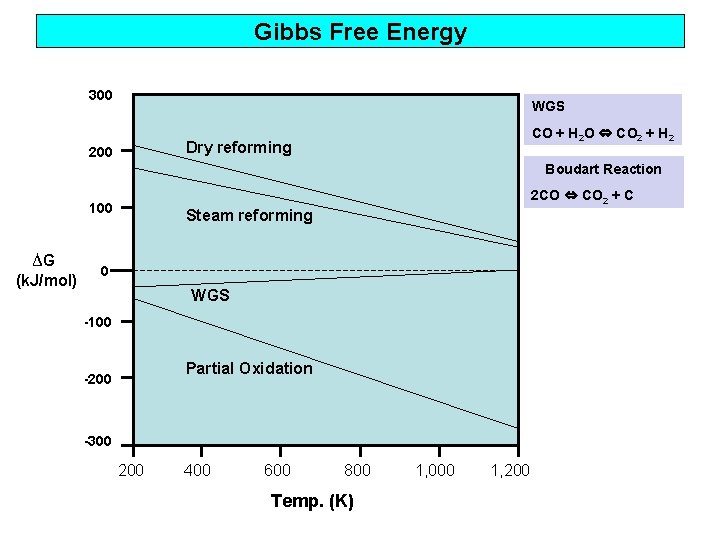
Gibbs Free Energy 300 WGS CO + H 2 O ⇔ CO 2 + H 2 Dry reforming 200 Boudart Reaction 2 CO ⇔ CO 2 + C 100 ∆G (k. J/mol) Steam reforming 0 WGS -100 Partial Oxidation -200 -300 200 400 600 800 Temp. (K) 1, 000 1, 200

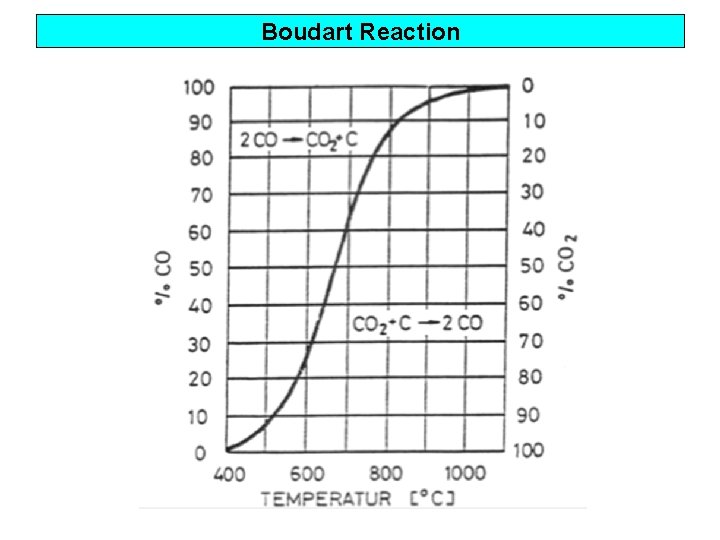
Boudart Reaction

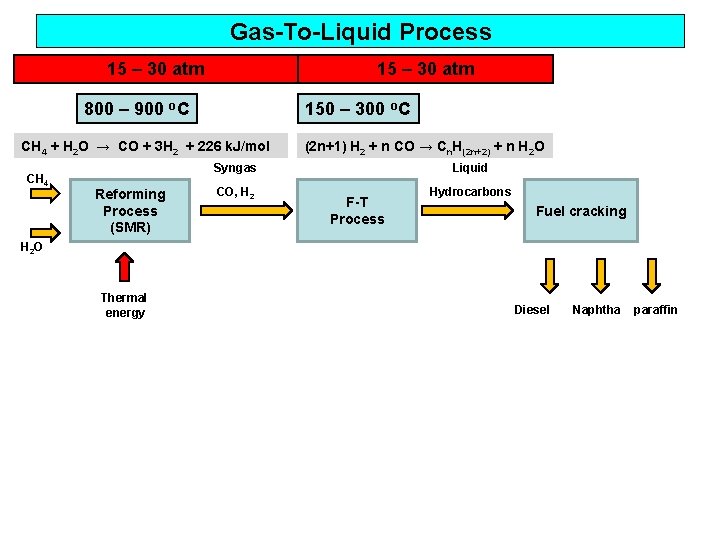
Gas-To-Liquid Process 15 – 30 atm 800 – 900 o. C 150 – 300 o. C CH 4 + H 2 O → CO + 3 H 2 + 226 k. J/mol CH 4 (2 n+1) H 2 + n CO → Cn. H(2 n+2) + n H 2 O Liquid Syngas Reforming Process (SMR) CO, H 2 F-T Process Hydrocarbons Fuel cracking H 2 O Thermal energy Diesel Naphtha paraffin

Gas-To-Liquid Process GTL Plant in Qatar

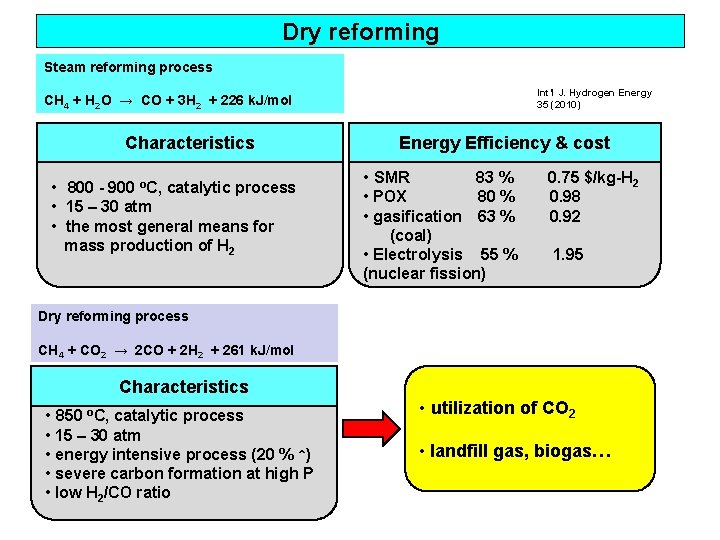
Dry reforming Steam reforming process Int’l J. Hydrogen Energy 35 (2010) CH 4 + H 2 O → CO + 3 H 2 + 226 k. J/mol Characteristics • 800 - 900 o. C, catalytic process • 15 – 30 atm • the most general means for mass production of H 2 Energy Efficiency & cost • SMR 83 % • POX 80 % • gasification 63 % (coal) • Electrolysis 55 % (nuclear fission) 0. 75 $/kg-H 2 0. 98 0. 92 1. 95 Dry reforming process CH 4 + CO 2 → 2 CO + 2 H 2 + 261 k. J/mol Characteristics • 850 o. C, catalytic process • 15 – 30 atm • energy intensive process (20 % ↑) • severe carbon formation at high P • low H 2/CO ratio • utilization of CO 2 • landfill gas, biogas…

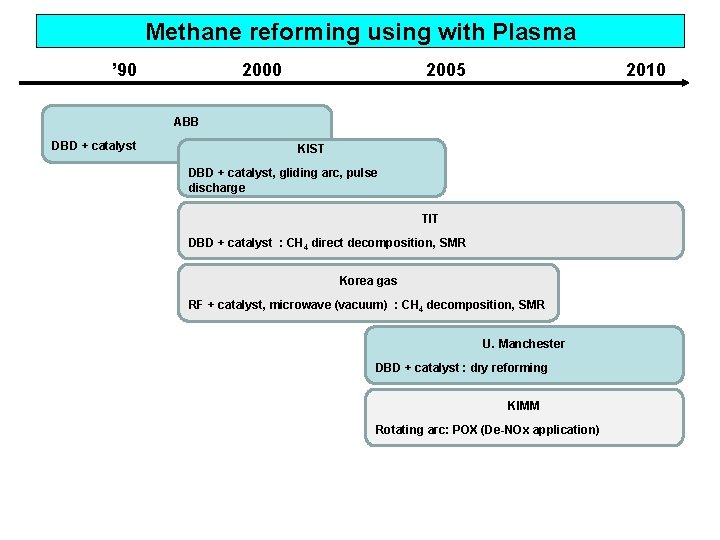
Methane reforming using with Plasma ’ 90 2005 2010 ABB DBD + catalyst KIST DBD + catalyst, gliding arc, pulse discharge TIT DBD + catalyst : CH 4 direct decomposition, SMR Korea gas RF + catalyst, microwave (vacuum) : CH 4 decomposition, SMR U. Manchester DBD + catalyst : dry reforming KIMM Rotating arc: POX (De-NOx application)

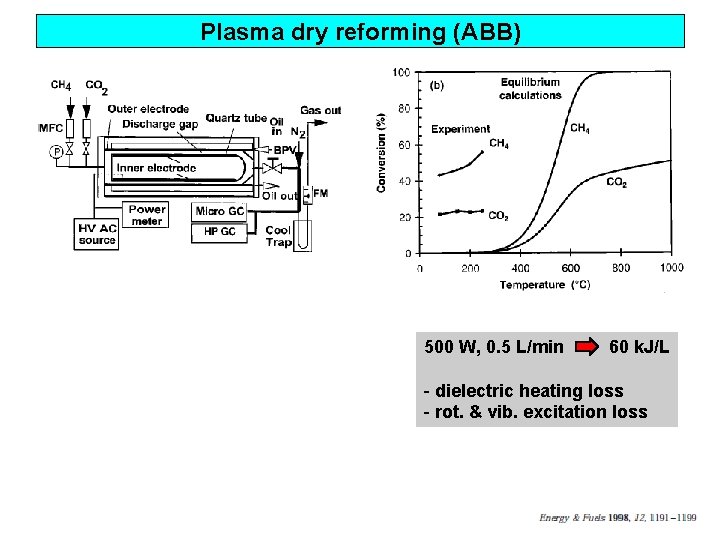
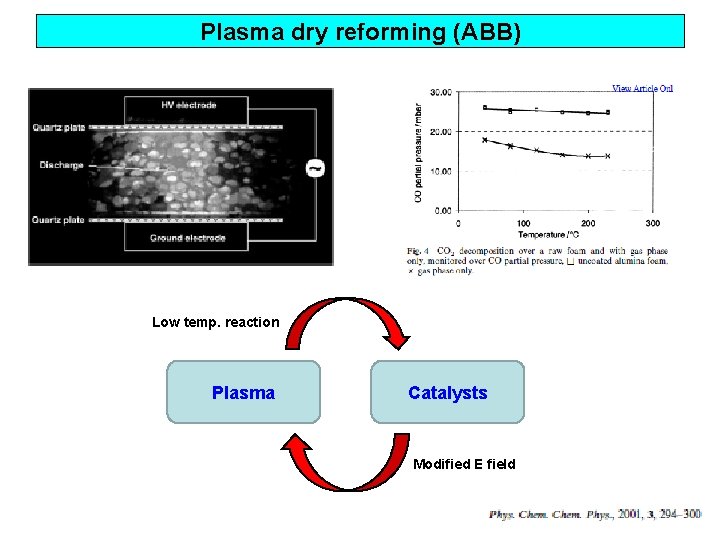
Plasma dry reforming (ABB) 500 W, 0. 5 L/min 60 k. J/L - dielectric heating loss - rot. & vib. excitation loss

Plasma dry reforming (ABB) Low temp. reaction Plasma Catalysts Modified E field

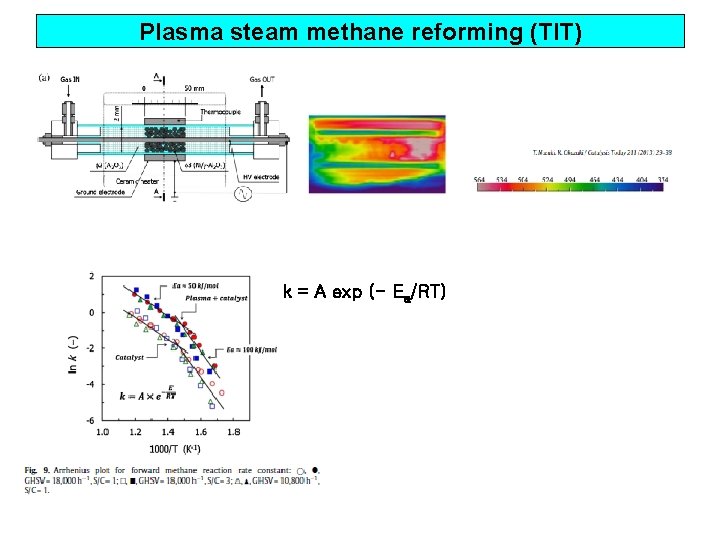
Plasma steam methane reforming (TIT) k = A exp (- Ea/RT)

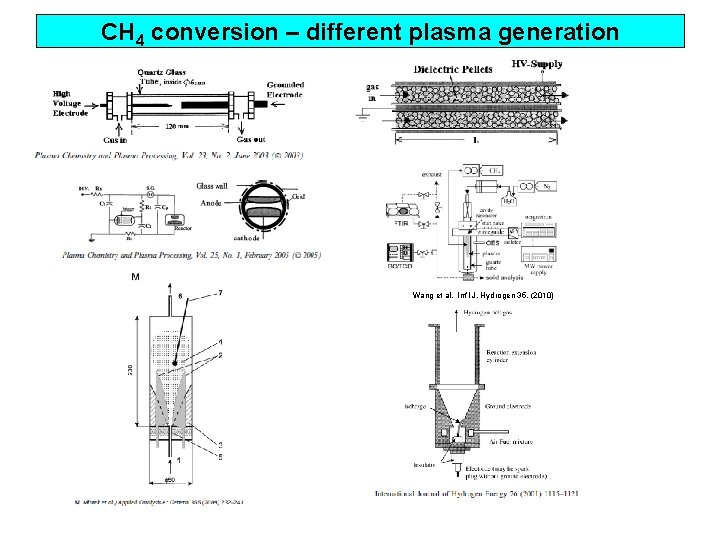
CH 4 conversion – different plasma generation Wang et al. Int’l J. Hydrogen 35, (2010)
![CH 4 conversion different plasma generation CH 4 conv SED KJL Type CH 4 conversion – different plasma generation CH 4 conv. [%] SED [KJ/L] Type](https://slidetodoc.com/presentation_image_h/a1abe442343be2048730c0caa5586692/image-32.jpg)
CH 4 conversion – different plasma generation CH 4 conv. [%] SED [KJ/L] Type Author Carrier gases major products 45 43 AC spark Y. Yang (1) CH 4 100 % C 2 H 2 25 43 DBD Y. Yang (1) CH 4 100 % C 2 H 6, C 3 H 8 12 6 gliding arc M. Mlotek et al. (2) CH 4 100 % C 2 H 2 22 60 DBD B. Eliasson CH 4 100 % C 2 H 6, C 3 H 8 32 39 CH 4 100 % C 2 H 6 100 75 (3) pulse corona (50 nsec) A. M. Ghorbanzadeh 1 plasmatron Bromberg et al. 5 microwave torch Wang et al. (4) (5) (6) (1) (2) (3) (4) (5) (6) CH 4/H 2 O, CH 4/O 2 CO, H 2 CH 4 (5%) + N 2 (95%) H 2, C, C 2 H 2 Plasma Chemistry Plasma Processing vol. 23, No. 2, 2003 Applied Catalyst A General 366, 232 -241, 2009 Plasma Chemistry Plasma Processing vol. 25, No. 1, 2005 Studies in Surface Science & Catalysis, vol 147, 577 -582, 2004 Int’l J. Hydrogen, 24 (1999) 1131 -1137 Int’l J. Hydrogen, 35 (2010) 135 -140 Methane conversion high temp. plasma > spark > pulsed corona > DBD Practical meaning of 1 J/L (DBD, SV: 50, 000 hr-1) 1 o. C ↑

Methane activation using with plasma direct process Heat + CO 2, H 2 O Indirect process CO: H 2 (1: 2) O 2 Ammonia Methanol O 2 C 2 H 6 C 2 H 4 O 2 CH 4 H 2 O CO: H 2 (1: 3) - H 2 ( < 300 o. C) CO 2 Formaldehyde (Room Temp. ) - CO CO: H 2 (1: 1) Hydrocarbons Syngas Acetic acid Phosgene Oxo-alcohols Metal carbonyls

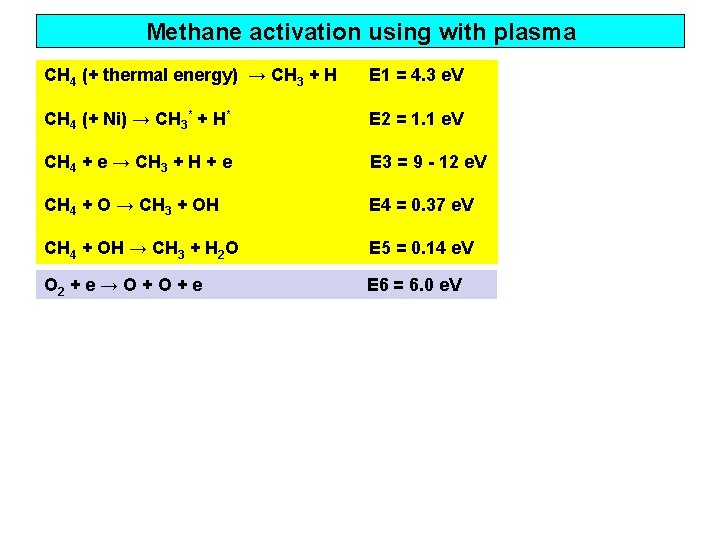
Methane activation using with plasma CH 4 (+ thermal energy) → CH 3 + H E 1 = 4. 3 e. V CH 4 (+ Ni) → CH 3* + H* E 2 = 1. 1 e. V CH 4 + e → CH 3 + H + e E 3 = 9 - 12 e. V CH 4 + O → CH 3 + OH E 4 = 0. 37 e. V CH 4 + OH → CH 3 + H 2 O E 5 = 0. 14 e. V O 2 + e → O + e E 6 = 6. 0 e. V

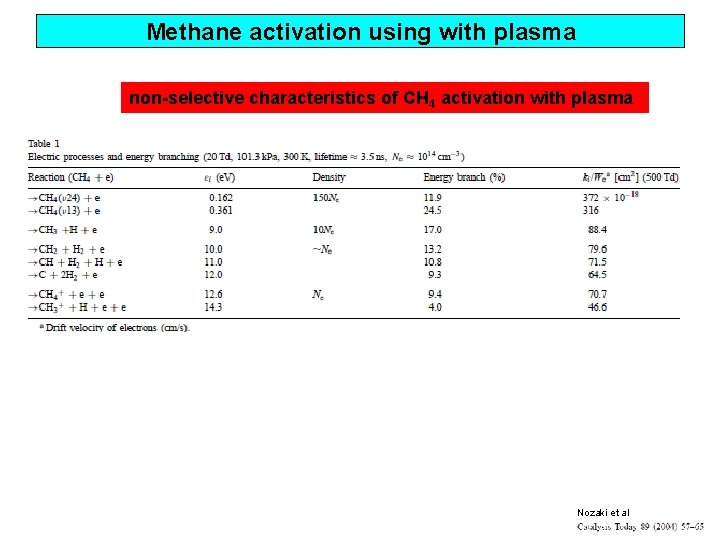
Methane activation using with plasma non-selective characteristics of CH 4 activation with plasma Nozaki et al

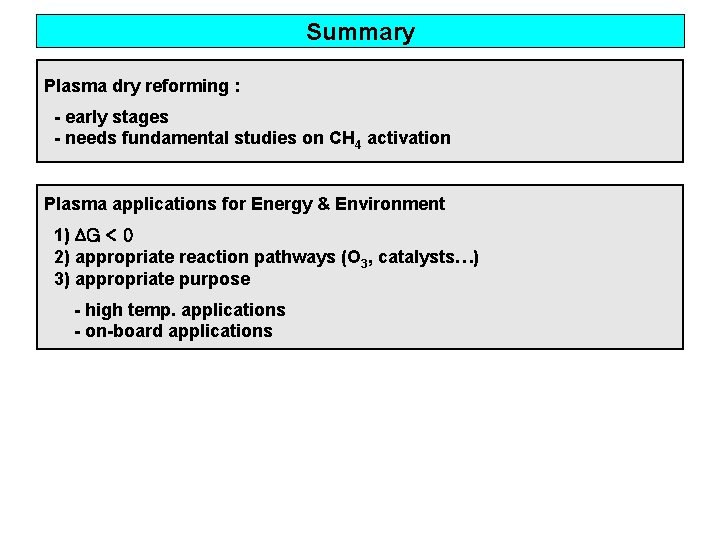
Summary Plasma dry reforming : - early stages - needs fundamental studies on CH 4 activation Plasma applications for Energy & Environment 1) ∆G < 0 2) appropriate reaction pathways (O 3, catalysts…) 3) appropriate purpose - high temp. applications - on-board applications