DRUGS USED IN DIABETES MELLITUS Classification of Diabetes

DRUGS USED IN DIABETES MELLITUS

Classification of Diabetes �Type 11 Diabetes �Diabetes due to secondary causes �Gestational Diabetes

Drugs used for diabetes all lower blood glucose INSULINS: only injectable preparations at present Other antidiabetes drugs: oral preparations Known as oral antidiabetes drugs/ Oral hypoglycaemic drugs

Oral antidiabetes drugs �Main drug classes can classified according to, �(How drugs have been developed) �Efficacy �Safety �Suitability �Availability �Cost �Drug interactions �New drug classes & their place in therapy

Main oral antidiabetes drug classes for type 2 diabetes Considering the effects in type 2 diabetes �Drugs to increase insulin secretion 1. Sulphonylureas �Drugs to improve insulin action (Insulin sensitivity) 2. Biguanides
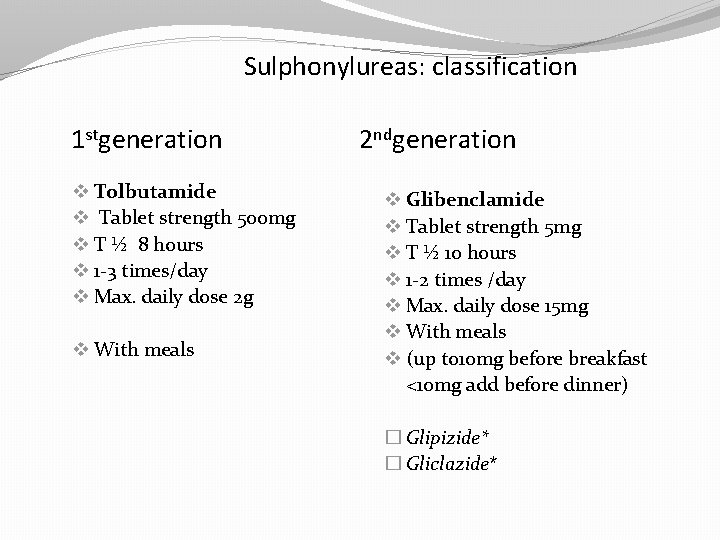
Sulphonylureas: classification 1 stgeneration v Tolbutamide v Tablet strength 500 mg v T ½ 8 hours v 1 -3 times/day v Max. daily dose 2 g v With meals 2 ndgeneration v Glibenclamide v Tablet strength 5 mg v T ½ 10 hours v 1 -2 times /day v Max. daily dose 15 mg v With meals v (up to 10 mg before breakfast <10 mg add before dinner) � Glipizide* � Gliclazide*
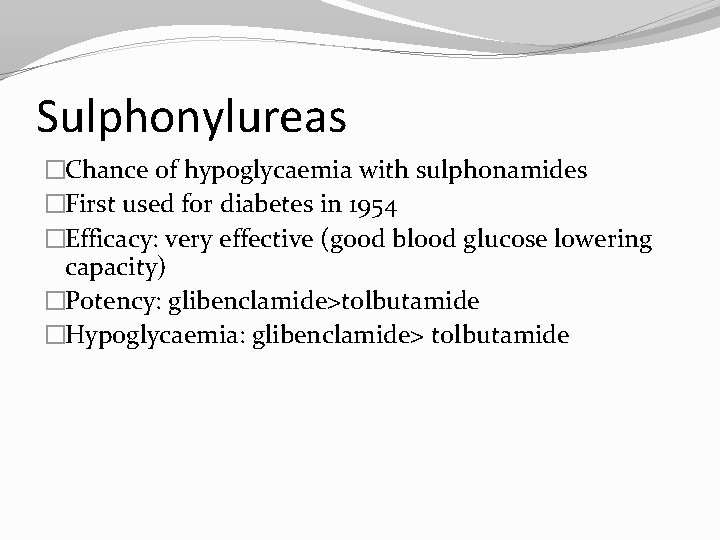
Sulphonylureas �Chance of hypoglycaemia with sulphonamides �First used for diabetes in 1954 �Efficacy: very effective (good blood glucose lowering capacity) �Potency: glibenclamide>tolbutamide �Hypoglycaemia: glibenclamide> tolbutamide
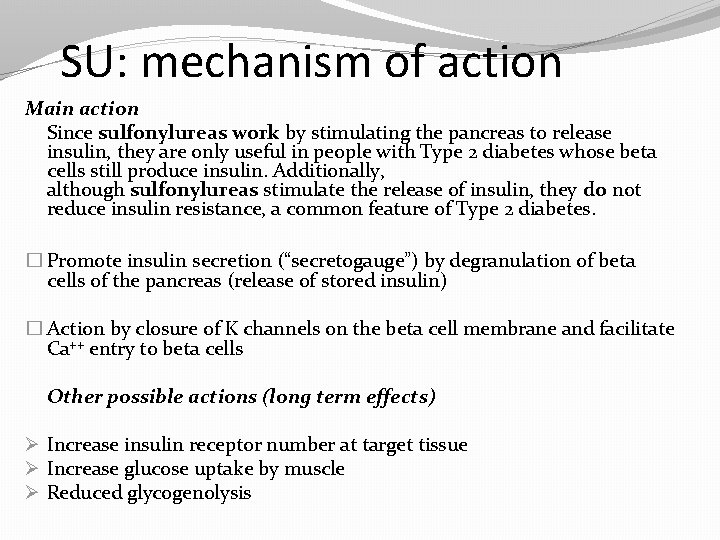
SU: mechanism of action Main action Since sulfonylureas work by stimulating the pancreas to release insulin, they are only useful in people with Type 2 diabetes whose beta cells still produce insulin. Additionally, although sulfonylureas stimulate the release of insulin, they do not reduce insulin resistance, a common feature of Type 2 diabetes. � Promote insulin secretion (“secretogauge”) by degranulation of beta cells of the pancreas (release of stored insulin) � Action by closure of K channels on the beta cell membrane and facilitate Ca++ entry to beta cells Other possible actions (long term effects) Ø Increase insulin receptor number at target tissue Ø Increase glucose uptake by muscle Ø Reduced glycogenolysis

Pharmacokinetics �Well absorbed from GIT �Highly protein bound �Metabolized in the liver �Excreted by the kidneys �Some drugs have active metabolites

Sulphonylureas: indications �Non obese type 2 diabetes: not responding to dietary therapy �Non obese Type 2 diabetes: presenting with a complication eg. a foot ulcer, UTI (together with dietary therapy)
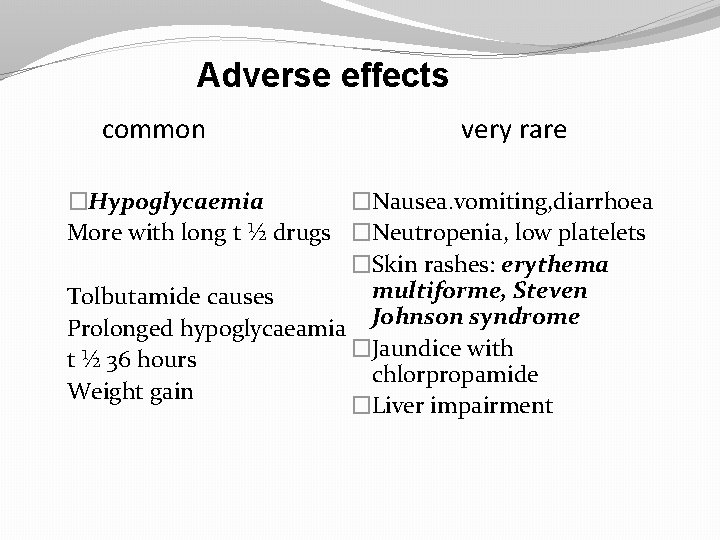
Adverse effects common very rare �Nausea. vomiting, diarrhoea �Hypoglycaemia More with long t ½ drugs �Neutropenia, low platelets �Skin rashes: erythema multiforme, Steven Tolbutamide causes Prolonged hypoglycaeamia Johnson syndrome �Jaundice with t ½ 36 hours chlorpropamide Weight gain �Liver impairment
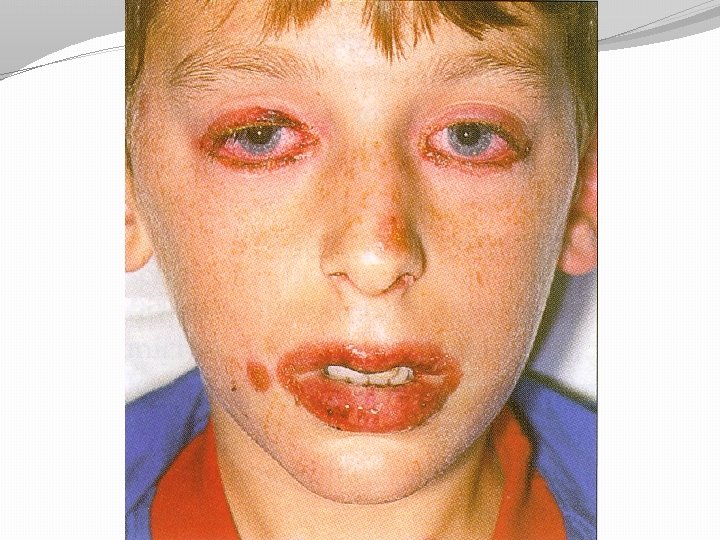
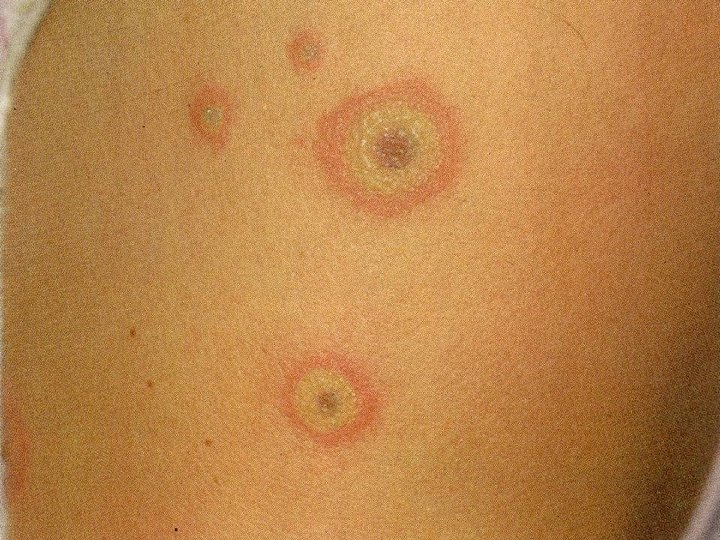

Contraindications / cautions �Type 1 diabetes �Pregnancy �Breast feeding �Liver disease �stressful states eg, severe infections, MI, surgery �Hyperglycaemic emergencies (DKA & HONK) Caution �Renal impairment �Elderly (tolbutamide has a short half life and is not excreted by the kidneys, hence is preferred to glibenclamide)

Sulphonylurea(SU) failure �Failure to lower blood glucose with SU Primary failure �If it occurs with in 1 month of starting therapy Secondary failure �due to beta cell exhaustion and failure to produce insulin and insulin resistance Insulin therapy is recommended for both types
Tolbutamide �Short acting �Metabolized in the liver �Safer in patients with renal impairment �Safer in elderly

Glibenclamide �Widely used �Has intermediate kinetics �Can be given as a single daily dose �Started with a daily dose of 5 mg in the morning before breakfast �Max dose is 15 mg/day

Chlorpropamide. �Longer duration of action �Risk of prolonged hypoglycaemia �Should not be used in elderly
Biguanide: metformin 500 mg/850 mg Mechanism of action �Increase glucose uptake by muscle in the presence of insulin �Increase insulin receptor number and affinity of target tissue �Inhibition of hepatic gluconeogenesis �Reduced intestinal glucose absorption �Reduced appetite and weight loss
�These effects are mediated by the initial activation by metformin of AMP-activated protein kinase (AMPK), a liver enzyme that plays an important role in insulin signaling, whole body energy balance, and the metabolism of glucose and fats. �Activation of AMPK is required for metformin's inhibitory effect on the production of glucose by liver cells. �Increased peripheral utilization of glucose may be due to improved insulin binding to insulin receptors. �Metformin administration also increases AMPK activity in skeletal muscle. �AMPK is known to cause GLUT 4 deployment to the plasma membrane, resulting in insulin-independent glucose uptake.

Metformin: Pharmacokinetics and indications �Well absorbed �Renal excretion (unchanged) Indications �Obese type 2 diabetes not responding to diet alone �Obese type 2 diabetes presenting with a complication such as UTI or a foot ulcer �Type 2 diabetes: when hypoglycaemia is a risk to life

Metformin: Adverse effects Common �Gastrointestinal disturbances �Anorexia , nausea, vomiting, diarrhoea �Malabsorption (B 12 absorption) Start with a low dose Immediately after meals 1 -3 times /day Max daily dose 3 g (1 gx 3) Rare �Lactic acidosis (A serious condition) �Hypoglycaemia (Very rare)
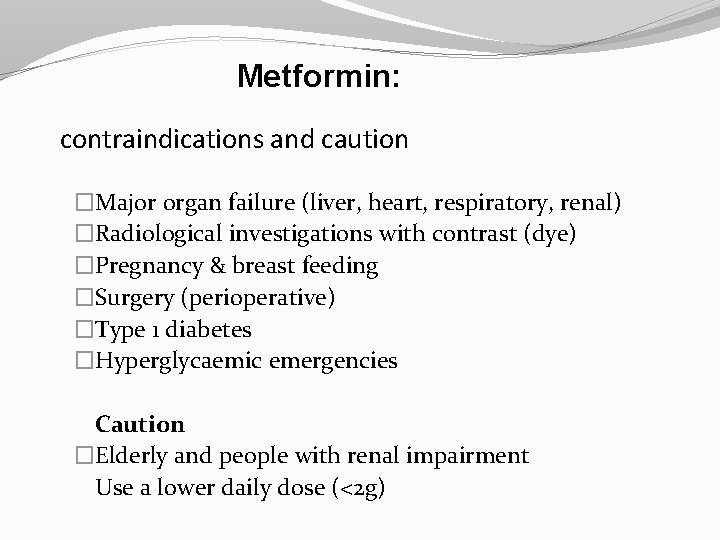
Metformin: contraindications and caution �Major organ failure (liver, heart, respiratory, renal) �Radiological investigations with contrast (dye) �Pregnancy & breast feeding �Surgery (perioperative) �Type 1 diabetes �Hyperglycaemic emergencies Caution �Elderly and people with renal impairment Use a lower daily dose (<2 g)
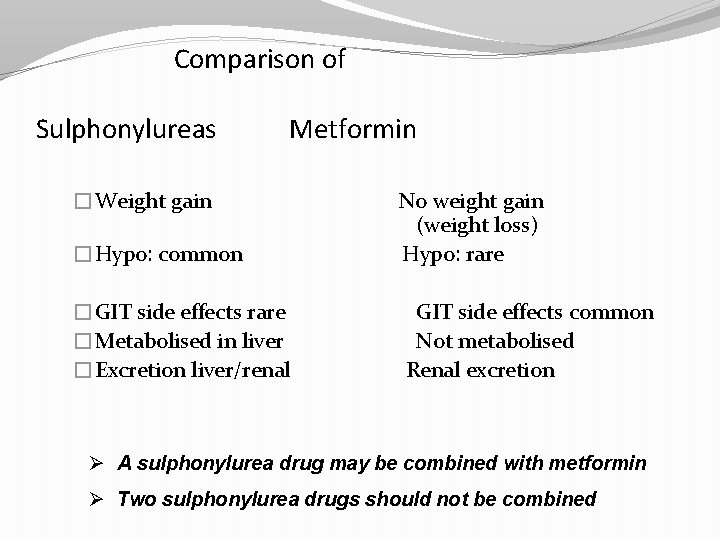
Comparison of Sulphonylureas Metformin �Weight gain �Hypo: common �GIT side effects rare �Metabolised in liver �Excretion liver/renal No weight gain (weight loss) Hypo: rare GIT side effects common Not metabolised Renal excretion Ø A sulphonylurea drug may be combined with metformin Ø Two sulphonylurea drugs should not be combined

New oral antidiabetes drugs �Alpha glucosidase inhibitors eg. acarbose Delayed conversion of disaccharides to monosaccharides Problems: intolerable GIT side effects Liver toxicity (hepatitis), monitor liver function Meglitinides: insulin secretogauges (non SU) eg repaglinide, nateglinide Thiozolidinediones: Improves insulin sensitivity eg pioglitazone, rosiglitazone Problems: Heart failure and liver failure
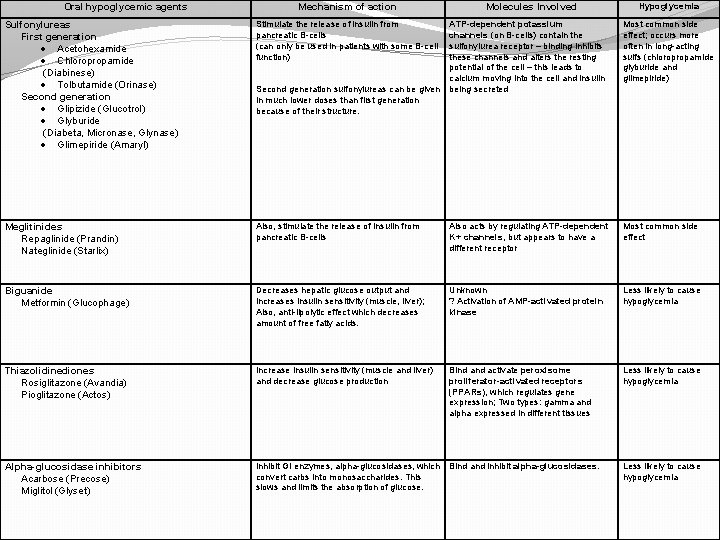
Oral hypoglycemic agents Mechanism of action Sulfonylureas First generation · Acetohexamide · Chloropropamide (Diabinese) · Tolbutamide (Orinase) Second generation · Glipizide (Glucotrol) · Glyburide (Diabeta, Micronase, Glynase) · Glimepiride (Amaryl) Stimulate the release of insulin from pancreatic B-cells (can only be used in patients with some B-cell function) Meglitinides Repaglinide (Prandin) Nateglinide (Starlix) Molecules Involved Hypoglycemia ATP-dependent potassium channels (on B-cells) contain the sulfonylurea receptor – binding inhibits these channels and alters the resting potential of the cell – this leads to calcium moving into the cell and insulin being secreted Most common side effect; occurs more often in long-acting sulfs (chloropropamide glyburide and glimepiride) Also, stimulate the release of insulin from pancreatic B-cells Also acts by regulating ATP-dependent K+ channels, but appears to have a different receptor Most common side effect Biguanide Metformin (Glucophage) Decreases hepatic glucose output and increases insulin sensitivity (muscle, liver); Also, anti-lipolytic effect which decreases amount of free fatty acids. Unknown ? Activation of AMP-activated protein kinase Less likely to cause hypoglycemia Thiazolidinediones Rosiglitazone (Avandia) Pioglitazone (Actos) Increase insulin sensitivity (muscle and liver) and decrease glucose production Bind activate peroxisome proliferator-activated receptors (PPARs), which regulates gene expression; Two types: gamma and alpha expressed in different tissues Less likely to cause hypoglycemia Alpha-glucosidase inhibitors Acarbose (Precose) Miglitol (Glyset) Inhibit GI enzymes, alpha-glucosidases, which convert carbs into monosaccharides. This slows and limits the absorption of glucose. Bind and inhibit alpha-glucosidases. Less likely to cause hypoglycemia Second generation sulfonylureas can be given in much lower doses than first generation because of their structure.
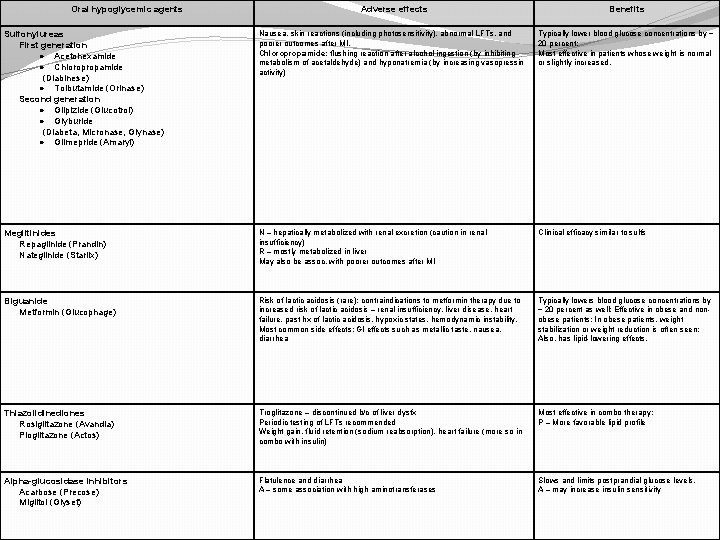
Oral hypoglycemic agents Adverse effects Benefits Sulfonylureas First generation · Acetohexamide · Chloropropamide (Diabinese) · Tolbutamide (Orinase) Second generation · Glipizide (Glucotrol) · Glyburide (Diabeta, Micronase, Glynase) · Glimepride (Amaryl) Nausea, skin reactions (including photosensitivity), abnormal LFTs, and poorer outcomes after MI. Chloropropamide: flushing reaction after alcohol ingestion (by inhibiting metabolism of acetaldehyde) and hyponatremia (by increasing vasopressin activity) Typically lower blood glucose concentrations by ~ 20 percent; Most effective in patients whose weight is normal or slightly increased. Meglitinides Repaglinide (Prandin) Nateglinide (Starlix) N – hepatically metabolized with renal excretion (caution in renal insufficiency) R – mostly metabolized in liver May also be assoc. with poorer outcomes after MI Clinical efficacy similar to sulfs Biguanide Metformin (Glucophage) Risk of lactic acidosis (rare): contraindications to metformin therapy due to increased risk of lactic acidosis – renal insufficiency, liver disease, heart failure, past hx of lactic acidosis, hypoxic states, hemodynamic instability. Most common side effects: GI effects such as metallic taste, nausea, diarrhea Typically lowers blood glucose concentrations by ~ 20 percent as well; Effective in obese and nonobese patients; In obese patients, weight stabilization or weight reduction is often seen; Also, has lipid-lowering effects. Thiazolidinediones Rosiglitazone (Avandia) Pioglitazone (Actos) Troglitazone – discontinued b/c of liver dysfx Periodic testing of LFTs recommended Weight gain, fluid retention (sodium reabsorption), heart failure (more so in combo with insulin) Most effective in combo therapy; P – More favorable lipid profile Alpha-glucosidase inhibitors Acarbose (Precose) Miglitol (Glyset) Flatulence and diarrhea A – some association with high aminotransferases Slows and limits postprandial glucose levels. A – may increase insulin sensitivity
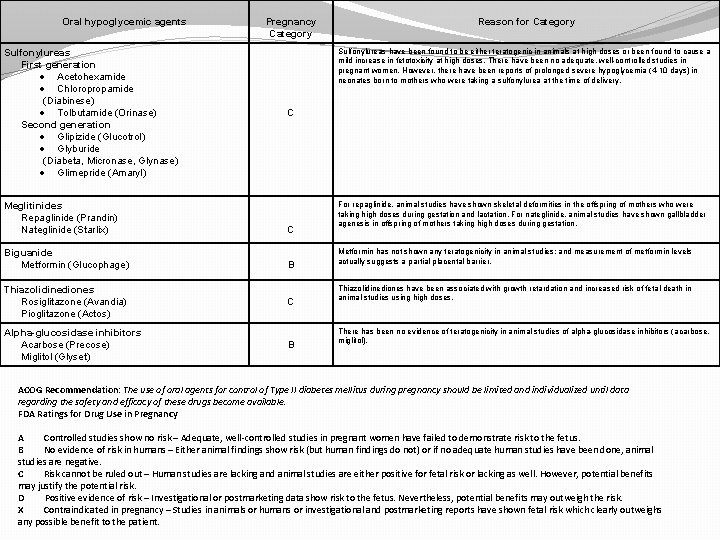
Oral hypoglycemic agents Sulfonylureas First generation · Acetohexamide · Chloropropamide (Diabinese) · Tolbutamide (Orinase) Second generation · Glipizide (Glucotrol) · Glyburide (Diabeta, Micronase, Glynase) · Glimepride (Amaryl) Meglitinides Repaglinide (Prandin) Nateglinide (Starlix) Biguanide Metformin (Glucophage) Pregnancy Category Reason for Category Sulfonylureas have been found to be either teratogenic in animals at high doses or been found to cause a mild increase in fetotoxicity at high doses. There have been no adequate, well-controlled studies in pregnant women. However, there have been reports of prolonged severe hypoglycemia (4 -10 days) in neonates born to mothers who were taking a sulfonylurea at the time of delivery. C C B Thiazolidinediones Rosiglitazone (Avandia) Pioglitazone (Actos) C Alpha-glucosidase inhibitors Acarbose (Precose) Miglitol (Glyset) B For repaglinide, animal studies have shown skeletal deformities in the offspring of mothers who were taking high doses during gestation and lactation. For nateglinide, animal studies have shown gallbladder agenesis in offspring of mothers taking high doses during gestation. Metformin has not shown any teratogenicity in animal studies; and measurement of metformin levels actually suggests a partial placental barrier. Thiazolidinediones have been associated with growth retardation and increased risk of fetal death in animal studies using high doses. There has been no evidence of teratogenicity in animal studies of alpha-glucosidase inhibitors (acarbose, miglitol). ACOG Recommendation: The use of oral agents for control of Type II diabetes mellitus during pregnancy should be limited and individualized until data regarding the safety and efficacy of these drugs become available. FDA Ratings for Drug Use in Pregnancy A Controlled studies show no risk – Adequate, well-controlled studies in pregnant women have failed to demonstrate risk to the fetus. B No evidence of risk in humans – Either animal findings show risk (but human findings do not) or if no adequate human studies have been done, animal studies are negative. C Risk cannot be ruled out – Human studies are lacking and animal studies are either positive for fetal risk or lacking as well. However, potential benefits may justify the potential risk. D Positive evidence of risk – Investigational or postmarketing data show risk to the fetus. Nevertheless, potential benefits may outweigh the risk. X Contraindicated in pregnancy – Studies in animals or humans or investigational and postmarketing reports have shown fetal risk which clearly outweighs any possible benefit to the patient.

Insulin �Polypeptide with 2 peptide chains �Linked by 2 disulphide Bonds �Metabolic activity is common to all mammalian species �Daily secretion 30 -40 units

Pharmacokinetics �Injected because digested if swallowed �Absorbed in to the blood inactivated in the liver & kidney. � 10% appear in urine. �T 1/2 is 5 min �Peak plasma concentration is in 30 -90 min
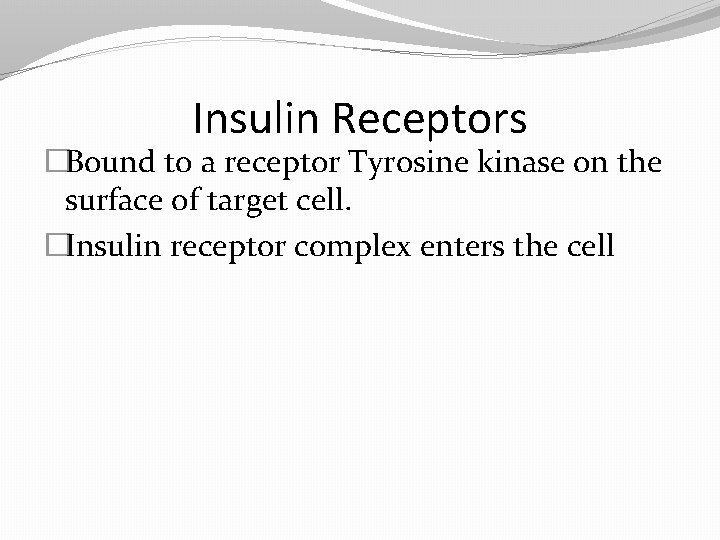
Insulin Receptors �Bound to a receptor Tyrosine kinase on the surface of target cell. �Insulin receptor complex enters the cell

Preparations of Insulins. �Source of Insulin(Human, Bovine, Porcine) �Formulation �Short acting �Intermediate acting �Long acting �Bi phasic

Short Duration Of action Insulins �Rapid onset of action �Soluble Insulin �Insulin Lispro

Intemediate duration of action �Isophane Insulin - A suspension with protamine �Insulin zinc suspension -Amorphous

Longer duration of action Insulin �Insulin Zinc suspension -Crystalline

Biphasic Insulins �Mixture of soluble insulin & Isophane insulin �Most commonly used ones are human Insulins �Soluble Insulin at 10 -50% of total Insulin concentration �Remove the need for patients to mix Insulin

Indications for use of Insulin. �Type 1 Diabetes mellitus �Type 11 Diabetes · Diabetic ketoacidosis · Non ketotic hyper osmolar coma · Surgery · Infections · Pregnancy

Side effects of Insulin �Hypoglycaemia �Warning signs due to Neuroglycopenia �Coma , Convulsions & Death �Allergic reactions �Lipoatrophy �Lipohypertrophy

Soluble Insulin �Short duration of action �Used 30 m before meals � 3 times a day �Colourless. �Is given I. V in diabetic ketoacidosis.

Insulin zinc suspensions �Amorphous �Crystalline

Dose of Insulins � 100 u/ml �Total daily output is 30 -40 units a day �Daily increment is 4 units a day. �A dose of over 100 u/day is due to noncompliance.

Advice to the patient �Don’t skip a meal �Signs of hypoglycaemia �Card �Sweet

Questions ?

THANK YOU
- Slides: 44