Drugs Structure and Properties Learning outcomes At the








- Slides: 32

Drugs – Structure and Properties

Learning outcomes • At the end of this section the student shall be able to: – differentiate acidic from basic drugs – predict ionization of a drug at different p. H – estimate acidity/basicity of a drug solution – predict solubility, absorption and distribution of a drug at different body compartment – discuss the effect of acidity, size, shape, and stereochemistry of a drug on its activity

Physicochemical properties of a drug § A drug molecule is a compound that has the ability to bind specifically to a receptor § It should also be absorbed, distributed, metabolized and excreted by the body § This depends on the physicochemical properties of a drug including 1. 2. 3. 4. 5. 6. 7. Acid-base property Water-lipid solubility Size Steric effect Conformational isomerism Optical isomerism Geometric isomerism

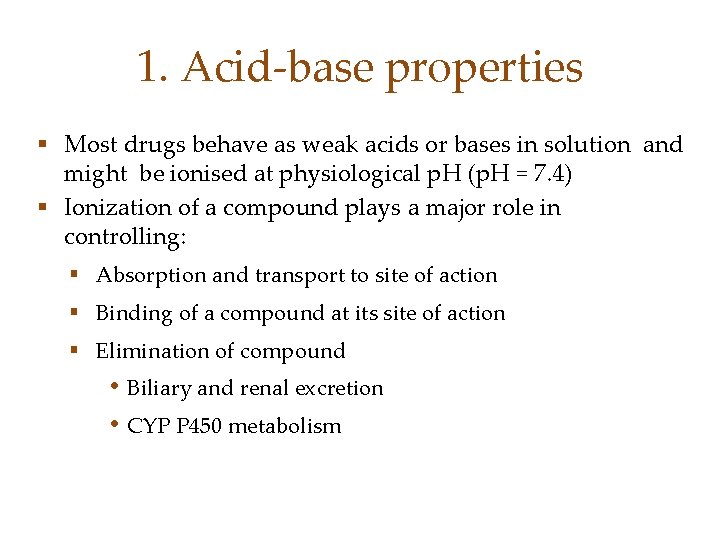
1. Acid-base properties § Most drugs behave as weak acids or bases in solution and might be ionised at physiological p. H (p. H = 7. 4) § Ionization of a compound plays a major role in controlling: § Absorption and transport to site of action § Binding of a compound at its site of action § Elimination of compound • Biliary and renal excretion • CYP P 450 metabolism

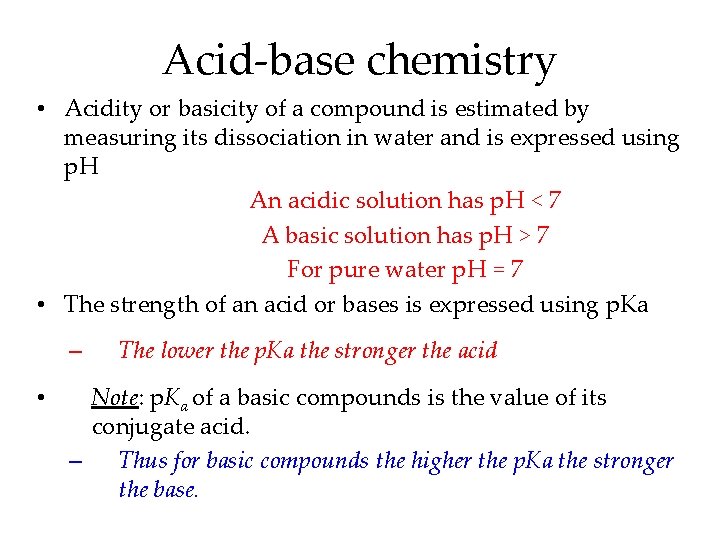
Acid-base chemistry • Acidity or basicity of a compound is estimated by measuring its dissociation in water and is expressed using p. H An acidic solution has p. H < 7 A basic solution has p. H > 7 For pure water p. H = 7 • The strength of an acid or bases is expressed using p. Ka – • The lower the p. Ka the stronger the acid Note: p. Ka of a basic compounds is the value of its conjugate acid. – Thus for basic compounds the higher the p. Ka the stronger the base.

Acid-base properties of drugs • For a drug molecule to be an acid or a base depends on the nature of its functional groups. – A drug molecule with a functional group that can donate hydrogen ion (H+) will be an acid – A drug molecule with a functional group that can accept hydrogen ion (H+) will be a base

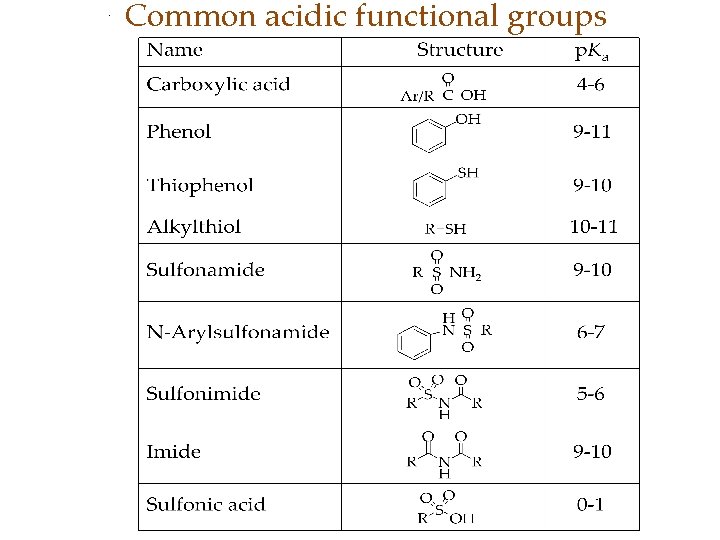
Common acidic functional groups

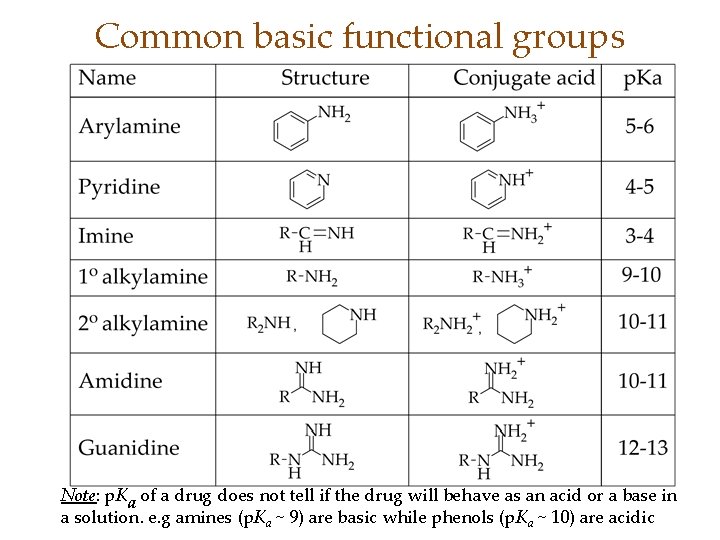
Common basic functional groups Note: p. Ka of a drug does not tell if the drug will behave as an acid or a base in a solution. e. g amines (p. Ka ~ 9) are basic while phenols (p. Ka ~ 10) are acidic

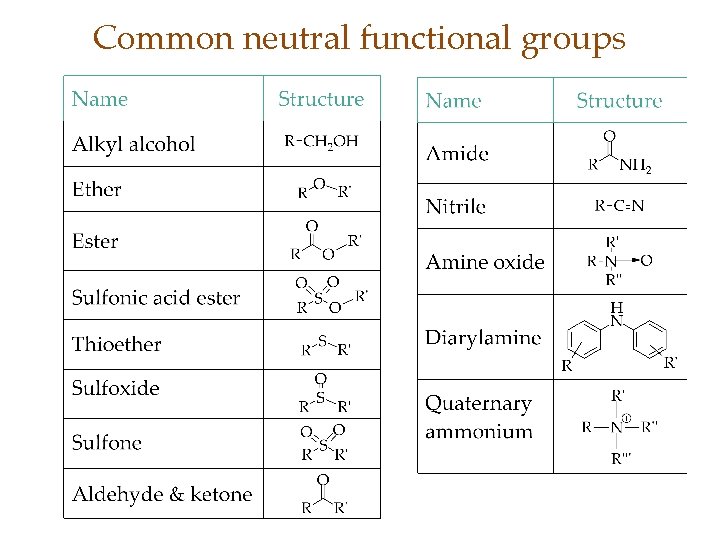
Common neutral functional groups

Acidity and basicity of salts • When a salt is hydrolyzed in water it dissociates completely to give anion and cation • The resulting solution can be basic or acidic depending on the constituents of the salts: Salt Acid/base property Example strong acid + strong base neutral Na. Cl strong acid + weak base acidic NH 4 Cl weak acid + strong base basic CH 3 COO-Na+ weak acid + weak base neutral CH 3 COO-NH 4+

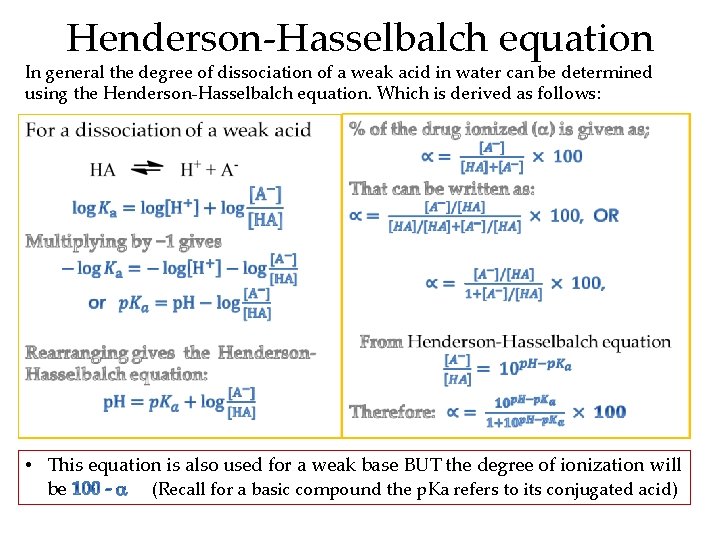
Henderson-Hasselbalch equation In general the degree of dissociation of a weak acid in water can be determined using the Henderson-Hasselbalch equation. Which is derived as follows: • • This equation is also used for a weak base BUT the degree of ionization will be (Recall for a basic compound the p. Ka refers to its conjugated acid)

Acidic/base Dissociation • Dissociation of acidic/basic molecule depends on their strength (i. e. p. Ka) and is affected by p. H of the medium • p. H = p. Ka compound is approximately 50% ionized • p. H < p. Ka + 1 compound is approximately fully unionized • p. H > p. Ka + 1 compound is approximately fully ionized • Recall: for a basic compounds the p. Ka is the value of its conjugate acid. – Thus in the intestine (p. H = 7 -8) weak acids will be ionized while weak bases will be neutral – Hence: weakly acidic oral drugs will have reduced absorption

2. Water-Lipid solubility § A successful drug must exhibit solubility to some extent in both water and lipid environments Because: – extremely water-soluble drugs may be unable to cross lipid barriers – very lipophilic drugs will be trapped in lipid and will not be able to reach their target quickly

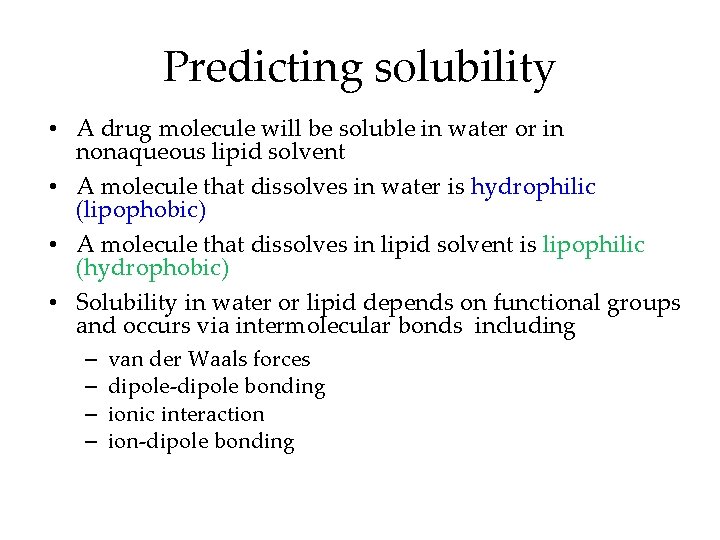
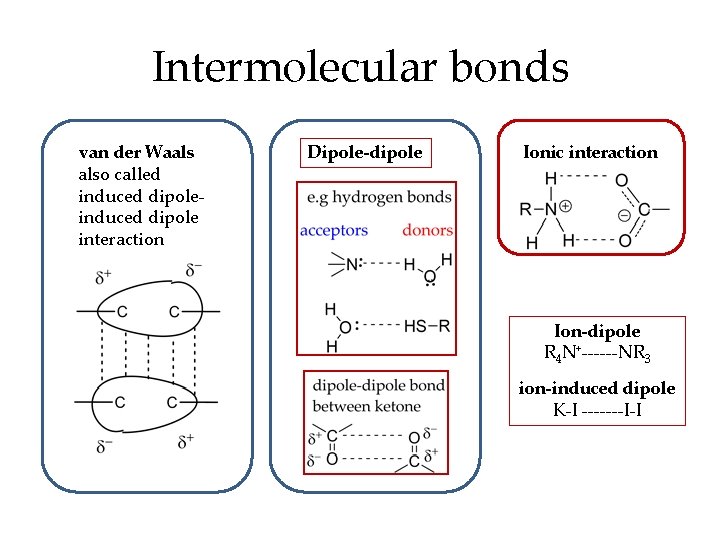
Predicting solubility • A drug molecule will be soluble in water or in nonaqueous lipid solvent • A molecule that dissolves in water is hydrophilic (lipophobic) • A molecule that dissolves in lipid solvent is lipophilic (hydrophobic) • Solubility in water or lipid depends on functional groups and occurs via intermolecular bonds including – – van der Waals forces dipole-dipole bonding ionic interaction ion-dipole bonding

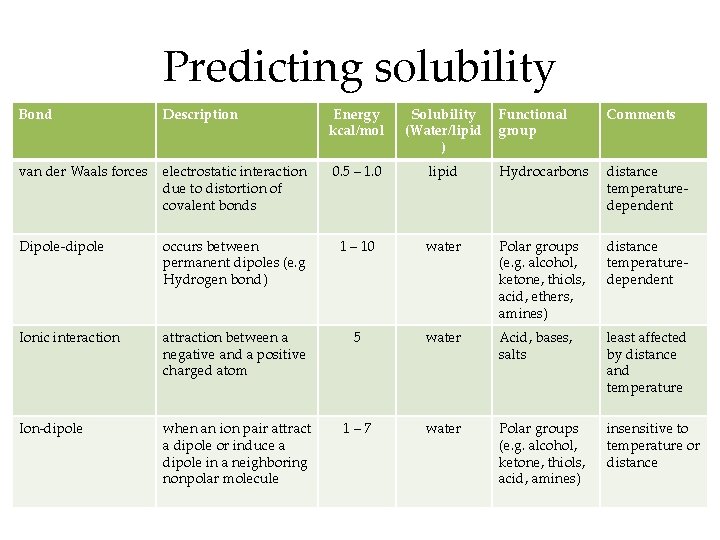
Predicting solubility Bond Description Energy kcal/mol Solubility (Water/lipid ) Functional group Comments van der Waals forces electrostatic interaction due to distortion of covalent bonds 0. 5 – 1. 0 lipid Hydrocarbons distance temperaturedependent Dipole-dipole occurs between permanent dipoles (e. g Hydrogen bond) 1 – 10 water Polar groups (e. g. alcohol, ketone, thiols, acid, ethers, amines) distance temperaturedependent Ionic interaction attraction between a negative and a positive charged atom 5 water Acid, bases, salts least affected by distance and temperature Ion-dipole when an ion pair attract a dipole or induce a dipole in a neighboring nonpolar molecule 1– 7 water Polar groups (e. g. alcohol, ketone, thiols, acid, amines) insensitive to temperature or distance

Intermolecular bonds van der Waals also called induced dipole interaction Dipole-dipole Ionic interaction Ion-dipole R 4 N+------NR 3 ion-induced dipole K-I -------I-I

Predicting solubility • Many drugs are poly-functional and can make all types of intermolecular interactions • Water/lipid solubility can be predicted by weighing the contribution of each functional group in the compound • There are two approaches for that: 1. Empirical: based on carbon solubilizing potential of functional groups 2. Quantitative: calculating log. P (log of partition coefficient)

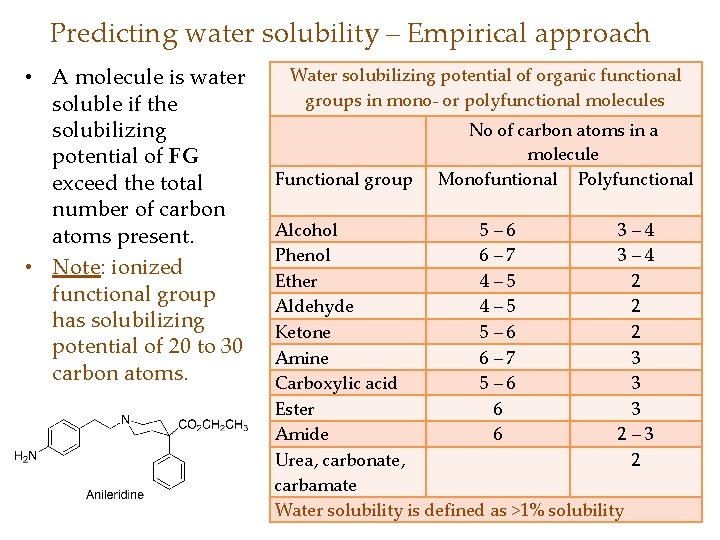
Predicting water solubility – Empirical approach • A molecule is water soluble if the solubilizing potential of FG exceed the total number of carbon atoms present. • Note: ionized functional group has solubilizing potential of 20 to 30 carbon atoms. Water solubilizing potential of organic functional groups in mono- or polyfunctional molecules Functional group No of carbon atoms in a molecule Monofuntional Polyfunctional Alcohol 5– 6 3– 4 Phenol 6– 7 3– 4 Ether 4– 5 2 Aldehyde 4– 5 2 Ketone 5– 6 2 Amine 6– 7 3 Carboxylic acid 5– 6 3 Ester 6 3 Amide 6 2– 3 Urea, carbonate, 2 carbamate Water solubility is defined as >1% solubility

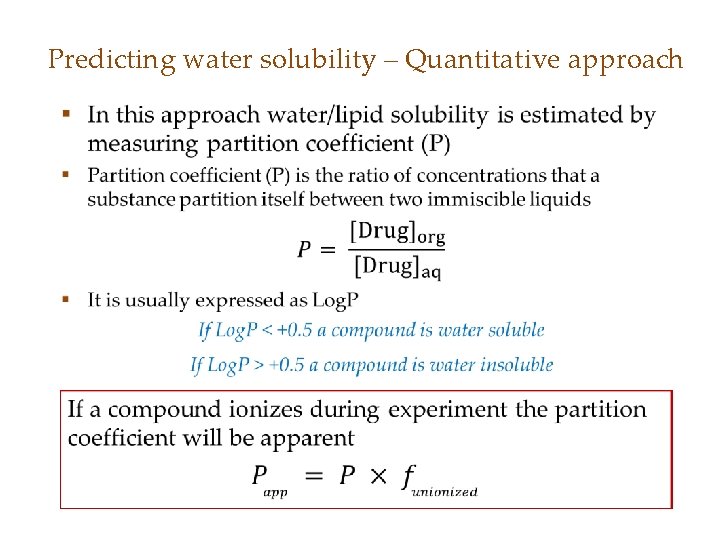
Predicting water solubility – Quantitative approach •

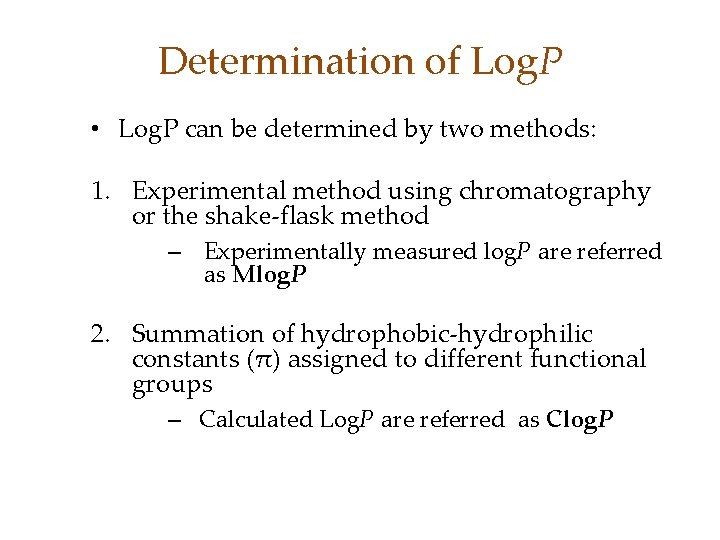
Determination of Log. P • Log. P can be determined by two methods: 1. Experimental method using chromatography or the shake-flask method – Experimentally measured log. P are referred as Mlog. P 2. Summation of hydrophobic-hydrophilic constants (π) assigned to different functional groups – Calculated Log. P are referred as Clog. P

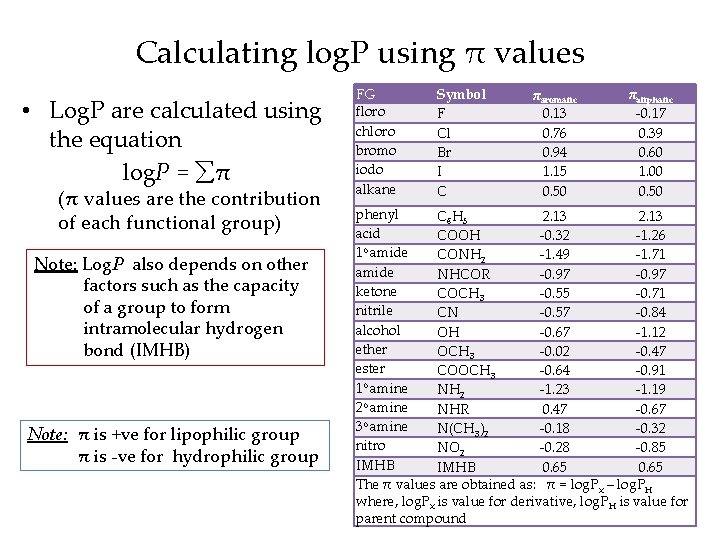
Calculating log. P using π values • Log. P are calculated using the equation log. P = π (π values are the contribution of each functional group) Note: Log. P also depends on other factors such as the capacity of a group to form intramolecular hydrogen bond (IMHB) Note: π is +ve for lipophilic group π is -ve for hydrophilic group FG floro chloro bromo iodo alkane Symbol F Cl Br I C πaromatic 0. 13 0. 76 0. 94 1. 15 0. 50 πaliphatic -0. 17 0. 39 0. 60 1. 00 0. 50 phenyl C 6 H 5 2. 13 acid COOH -0. 32 -1. 26 1 o amide CONH 2 -1. 49 -1. 71 amide NHCOR -0. 97 ketone COCH 3 -0. 55 -0. 71 nitrile CN -0. 57 -0. 84 alcohol OH -0. 67 -1. 12 ether OCH 3 -0. 02 -0. 47 ester COOCH 3 -0. 64 -0. 91 1 o amine NH 2 -1. 23 -1. 19 o 2 amine NHR 0. 47 -0. 67 o 3 amine N(CH 3)2 -0. 18 -0. 32 nitro NO 2 -0. 28 -0. 85 IMHB 0. 65 The π values are obtained as: π = log. Px – log. PH where, log. Px is value for derivative, log. PH is value for parent compound

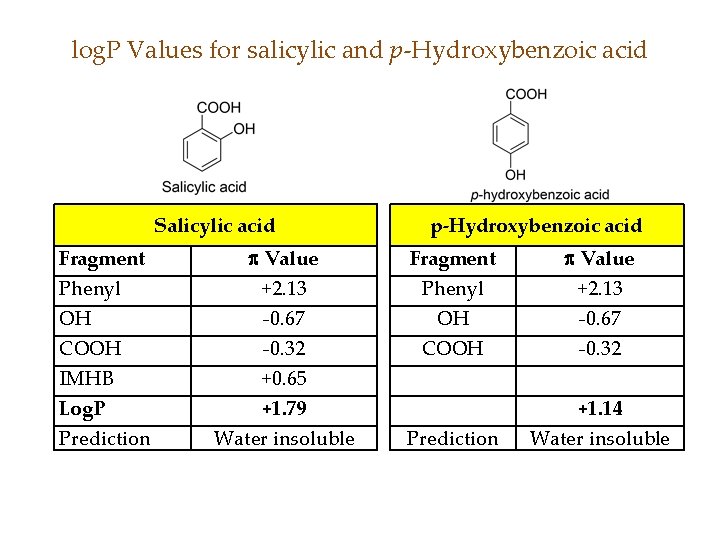
log. P Values for salicylic and p-Hydroxybenzoic acid Salicylic acid Fragment Phenyl OH COOH IMHB Log. P Prediction Value +2. 13 -0. 67 -0. 32 +0. 65 +1. 79 Water insoluble p-Hydroxybenzoic acid Fragment Phenyl OH COOH Value +2. 13 -0. 67 -0. 32 Prediction +1. 14 Water insoluble

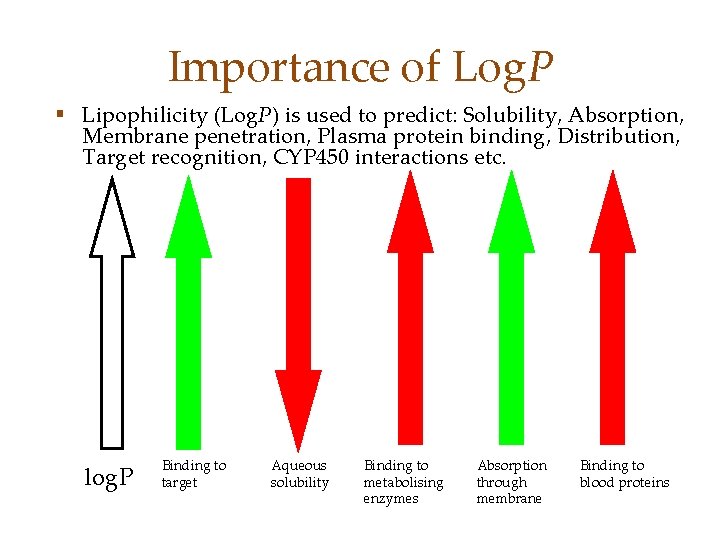
Importance of Log. P § Lipophilicity (Log. P) is used to predict: Solubility, Absorption, Membrane penetration, Plasma protein binding, Distribution, Target recognition, CYP 450 interactions etc. log. P Binding to target Aqueous solubility Binding to metabolising enzymes Absorption through membrane Binding to blood proteins

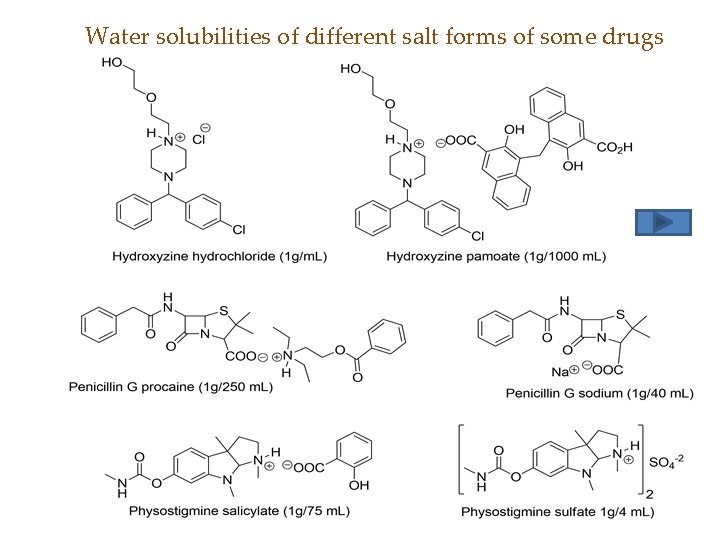
Solubility of salts • Solubility of organic salt in water depends on the degree of its dissociation – Salt of strong acid/strong base > strong acid/weak base > weak acid/weak base • Also on molecular weight, high molecular weight salts are less soluble in water

Water solubilities of different salt forms of some drugs

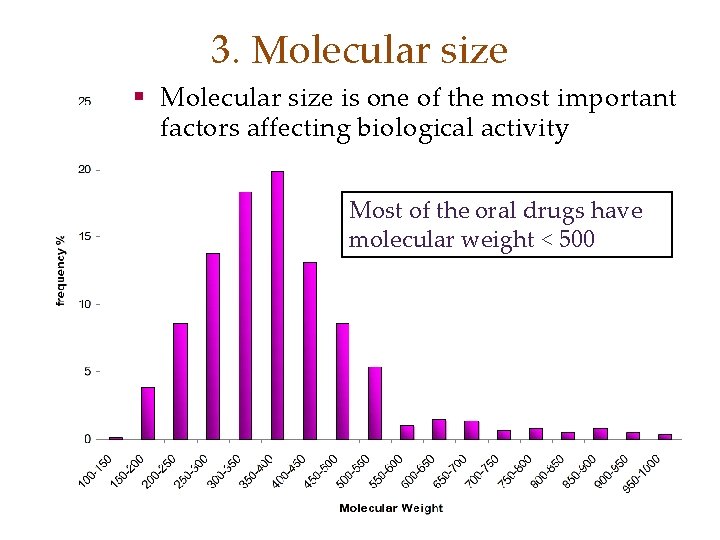
3. Molecular size § Molecular size is one of the most important factors affecting biological activity Most of the oral drugs have molecular weight < 500

4. Steric Effects § Bulky substituent appended close to pharmacophore may impede the geometry of interaction between a drug and its receptor § Steric effect is estimated by The Taft steric parameter (Es)

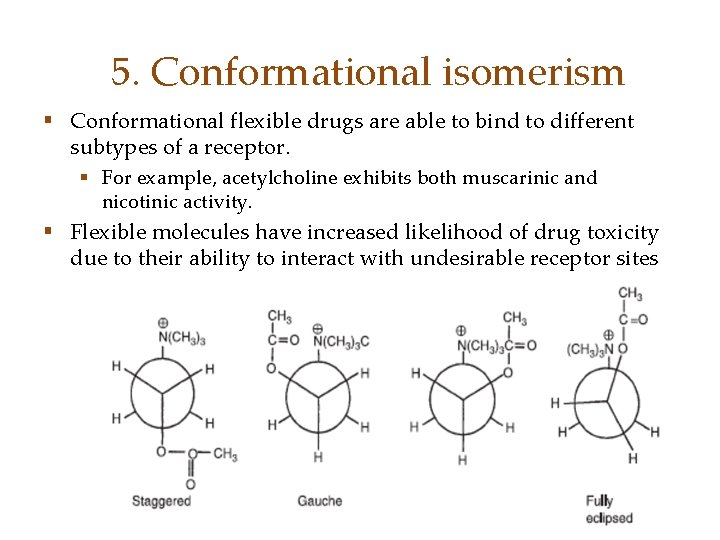
5. Conformational isomerism § Conformational flexible drugs are able to bind to different subtypes of a receptor. § For example, acetylcholine exhibits both muscarinic and nicotinic activity. § Flexible molecules have increased likelihood of drug toxicity due to their ability to interact with undesirable receptor sites

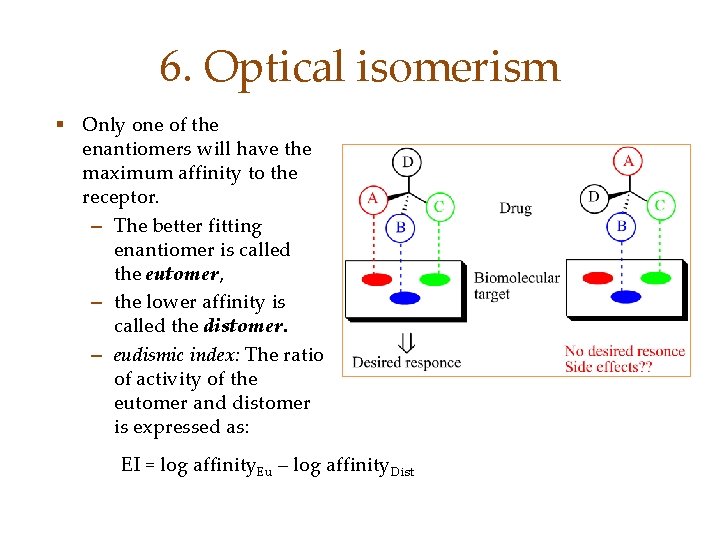
6. Optical isomerism § Only one of the enantiomers will have the maximum affinity to the receptor. – The better fitting enantiomer is called the eutomer, – the lower affinity is called the distomer. – eudismic index: The ratio of activity of the eutomer and distomer is expressed as: EI = log affinity. Eu – log affinity. Dist

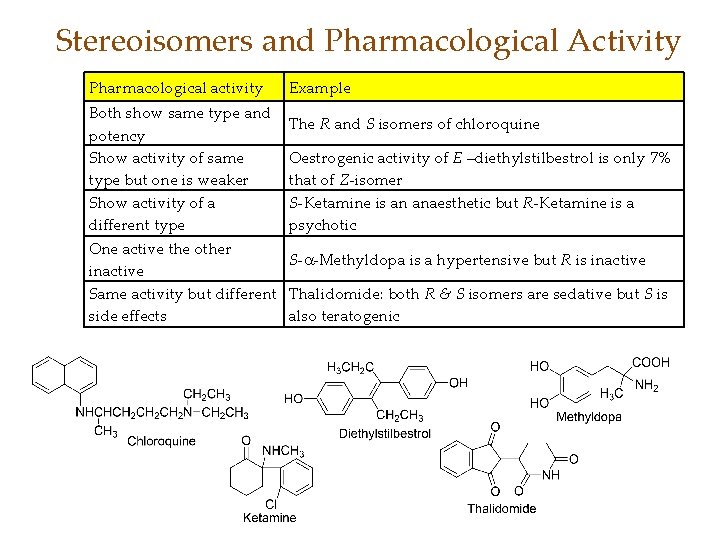
Stereoisomers and Pharmacological Activity Pharmacological activity Both show same type and potency Show activity of same type but one is weaker Show activity of a different type One active the other inactive Same activity but different side effects Example The R and S isomers of chloroquine Oestrogenic activity of E –diethylstilbestrol is only 7% that of Z-isomer S-Ketamine is an anaesthetic but R-Ketamine is a psychotic S- -Methyldopa is a hypertensive but R is inactive Thalidomide: both R & S isomers are sedative but S is also teratogenic

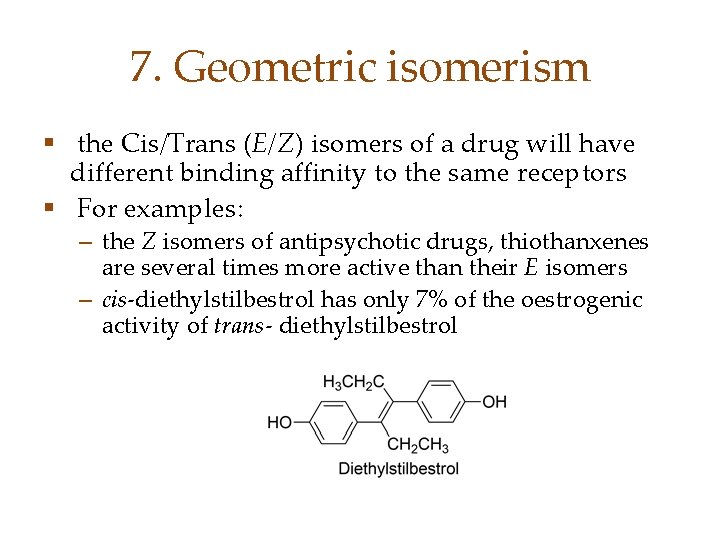
7. Geometric isomerism § the Cis/Trans (E/Z) isomers of a drug will have different binding affinity to the same receptors § For examples: – the Z isomers of antipsychotic drugs, thiothanxenes are several times more active than their E isomers – cis-diethylstilbestrol has only 7% of the oestrogenic activity of trans- diethylstilbestrol

Lipinski’s rule of five § Druggable molecule should possess these characteristics: – A molecular weight of less than 500 – A log. P value of less than 5 – Few than 5 H-bonding donors (Sum of NH and OH) – Less than 10 H-bonding acceptors (Sum of N and O)