DrugInduced Acute Kidney Injury Medications Mechanisms of Injury













- Slides: 36

Drug-Induced Acute Kidney Injury Medications, Mechanisms of Injury, and Management

Learning Objectives u. Review common medications implicated in acute kidney injury (AKI) and their mechanisms of nephrotoxicity u. Differentiate between clinical presentations and risk factors of drug-induced AKI u. Outline strategies used to prevent and manage druginduced AKI 2

Epidemiology u. AKI is reported to occur in up to 7% of hospitalized patients and 20 -30% of critically ill patients, with 6% eventually requiring renal replacement therapy u. Drugs have been implicated in up to 60% of in-hospital AKI cases and 19 -25% of cases of severe acute renal failure 3

Drug-Induced AKI: Classification v Hemodynamically-Mediated Kidney Injury u. ACE Inhibitors u. NSAIDs u. Calcineurin Inhibitors v Tubuloepithelial Injury & Tubulointerstitial Nephritis u. Acute Tubular Necrosis (ATN) u. Acute Interstitial Nephritis (AIN) v Crystal Nephropathy u. Direct Intratubular Obstruction & Nephrolithiasis u. Indirect Intratubular Obstruction 4

Hemodynamically-Mediated Kidney Injury Prerenal Injury 5

Hemodynamically Mediated Kidney Injury u“Prerenal” injury related to reduced renal blood flow (i. e. hypovolemia, CHF, bleeding, sepsis, ascites) u. Injury results from decreased tissue perfusion and decrease in GFR u. Normally, the kidney attempts to maintain the GFR by altering renal blood flow via prostaglandins (afferent) and angiotensin II (efferent arteriole) u. The insult is exacerbated when this response is inhibited by medications (i. e. ACEIs/ARBs and NSAIDs) 6

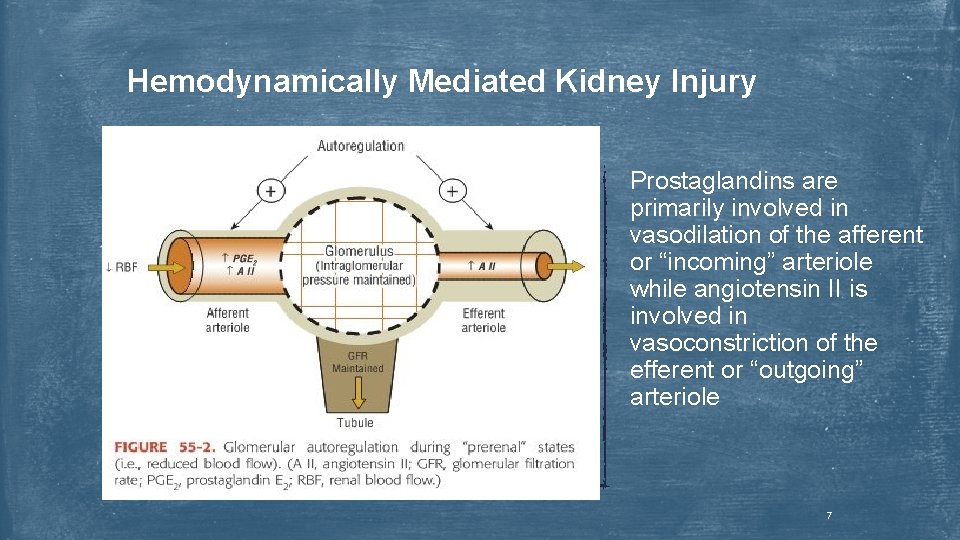
Hemodynamically Mediated Kidney Injury Prostaglandins are primarily involved in vasodilation of the afferent or “incoming” arteriole while angiotensin II is involved in vasoconstriction of the efferent or “outgoing” arteriole 7

NSAIDs u Unlikely to affect renal function in the absence of diminished renal perfusion u Mechanism: ↓ prostaglandin synthesis → afferent arteriole vasoconstriction → ↓ glomerular pressure → ↓ GFR u Clinical Presentation: u ↓ urine output u ↑ edema, BUN, Scr, K+, blood pressure u Fractional excretion Na < 1% u Risk Factors: age > 60 years, CKD, heart failure, concurrent nephrotoxic medications, and hepatic disease with ascites 8

NSAIDs u Prevention: u. Use alternative analgesics u. Use low-dose/short duration treatment u. Avoid potent NSAIDs (i. e. indomethacin) u. Avoid ACEIs/ARBs and diuretics in high-risk or dehydrated patients u. Appropriate monitoring (Scr, BUN, etc. ) u Management: u. Discontinue NSAID u. Recovery is rapid and baseline function is usually restored 9

Angiotensin Converting Enzyme Inhibitors & Angiotensin Receptor Blockers u Mechanism: ↓ angiotensin II production/action → efferent arteriole vasodilation → ↓ glomerular pressure → ↓ GFR u Clinical Presentation: u. Moderate vs. detrimental rise in serum creatinine u. Moderate: ↑ Scr ≤ 30% within 3 -5 days of initiation with stabilization in 1 -2 weeks is expected and reasonable u. Detrimental: ↑ Scr > 30% within 1 -2 weeks of initiation u Risk Factors: renal artery stenosis, volume depletion, heart failure, CKD including diabetic nephropathy 10

Angiotensin Converting Enzyme Inhibitors & Angiotensin Receptor Blockers u Prevention: u. Recognize patients at highest risk u. Initiate at very low doses u. Titrate every 2 -4 weeks as opposed to every 3 -5 days u. Avoid NSAIDs and diuretics in high-risk or dehydrated patients u. Appropriate monitoring (Scr, K+, etc. ) u Management: u. Discontinue ACEI/ARB (reinitiate once volume is corrected or at a point where the diuretic dose can be decreased) u. Manage hyperkalemia accordingly u. Baseline function is usually restored several days after discontinuation 11

Calcineurin Inhibitors u The nephrotoxic potential of cyclosporine and tacrolimus complicates their use, as they are the most common immunosuppressive agents used in kidney transplantation u Mechanism: ↑ renal vasoconstriction (thromboxane A 2, endothelin, RAAS) + ↓ renal vasodilation (prostaglandins) → afferent vasoconstriction → ↓ glomerular pressure → ↓ GFR u Clinical Presentation: u↓ urine output u↑ Scr, blood pressure, K+ u. Sodium retention 12

Calcineurin Inhibitors u Risk Factors: age > 65 yrs, high dose, concurrent nephrotoxic drugs (diuretics, NSAIDs), interactions that ↑ calcineurin inhibitor concentrations (CYP 3 A 4 inhibitors) u Prevention: u. Therapeutic drug monitoring of cyclosporine/tacrolimus u. Decreased dose (balance nephrotoxicity with risk of graft rejection) u. Appropriate monitoring (Scr, BUN, etc. ) u Management: u. Treat contributing illness and/or remove interacting drug u. Switch immunosuppressant if nephrotoxicity is progressive/severe 13

Tubuloepithelial Injury & Tubulointerstitial Nephritis Intrarenal Injury 14

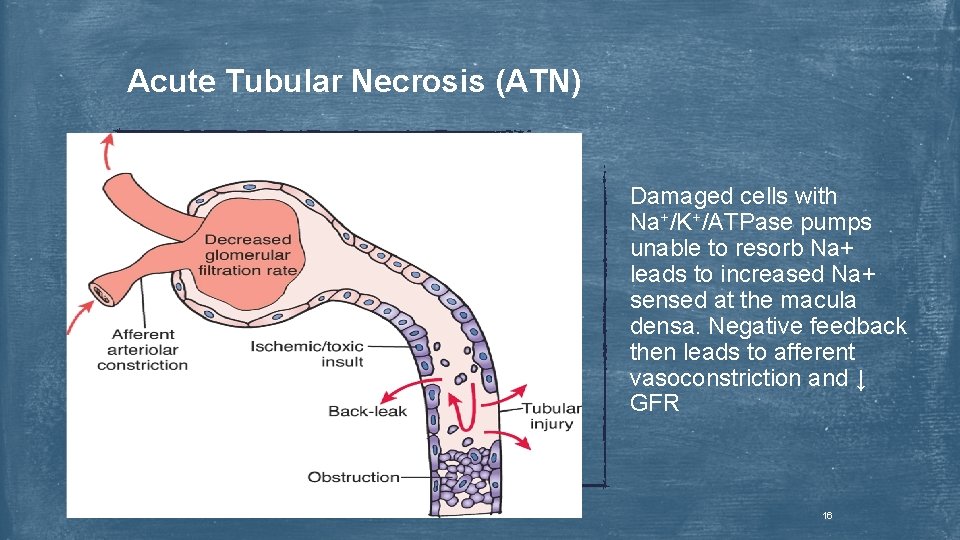
Acute Tubular Necrosis (ATN) u “Intrarenal” injury involving ischemia or cellular injury due endogenous toxins (i. e. myoglobin), or exogenous toxins (i. e. aminoglycosides) u Direct cellular toxicity or ischemia leads to cellular degeneration and sloughing from the proximal and/or distal tubules → inability to reabsorb electrolytes, ↓ GFR, tubular obstruction u Urine contains cellular debris/cast and will appear muddy-brown often without evidence of hematuria u Oliguric phase (2 -3 weeks) is often followed by tubular regeneration or a recovery phase (2 -3 weeks) 15

Acute Tubular Necrosis (ATN) Damaged cells with Na+/K+/ATPase pumps unable to resorb Na+ leads to increased Na+ sensed at the macula densa. Negative feedback then leads to afferent vasoconstriction and ↓ GFR 16

Aminoglycosides u Gentamicin, Tobramycin, Neomycin, Amikacin u Nephrotoxicity occurs in up to 10 -25% of patients undergoing a therapeutic course u Aminoglycosides are non-protein bound medications primarily excreted by glomerular filtration u Toxicity is a result of their cationic charge, facilitating their binding to negatively charged tubular epithelium phospholipids and intracellular lysosomal transport u Most cationic (and therefore toxic) → least cationic u. Neomycin > tobramycin, gentamicin, amikacin > streptomycin 17

Aminoglycosides u. Mechanism: uptaken by proximal tubule → ↑ reactive oxygen species → mitochondrial injury → cellular necrosis u. Clinical Presentation: Within 5 -10 days of initiation u↑ Scr, BUN, urine electrolytes u. Typically non-oliguric (urine > 500 m. L/d) u. Mild proteinuria (< 1 g/d) u. Risk Factors: ↑ dose/duration/trough concentration, concurrent nephrotoxic drugs (i. e. cyclosporine, diuretics, NSAIDs, vancomycin), patient related factors (↑ age, diabetes, CKD, dehydration, shock, liver disease) 18

Aminoglycosides u Prevention: u Alternate antibiotics if possible u Limit total aminoglycoside dose and duration (< 7 days if possible) u Extended interval dosing (once daily) associated with less nephrotoxicity than traditional dosing (TID) – 0 -5% vs. 17% u Renal tubule accumulation is saturated during peak concentrations u Avoid volume depletion u Avoid concurrent nephrotoxic drugs u Management: u Discontinue aminoglycoside or alter regimen u Discontinue other nephrotoxic drugs if possible u Maintain adequate hydration u Kidney injury is generally reversible after discontinuation 19

Amphotericin B u Nephrotoxicity related to amphotericin B is associated with the cumulative dose administered u It is estimated that approximately 80% of patients treated with amphotericin B will develop some renal dysfunction u Toxicity is related to a combination of direct proximal tubular cell toxicity and afferent arteriole vasoconstriction u Liposomal formulations are able to reduce direct amphotericin B interaction with tubular epithelial cell membranes 20

Amphotericin B u Clinical Presentation: u↑ Scr, BUN, urine electrolytes u. Typically non-oliguric (urine > 500 m. L/d) u. Impaired urinary concentrating ability u Risk Factors: large cumulative doses, pre-existing kidney disease, volume depletion, ↑ age, concurrent use of diuretics or nephrotoxic drugs (i. e. cyclosporine) 21

Amphotericin B u Prevention: u. Use the liposomal formulation in high risk patients or an alternative antifungal agent if possible (i. e. voriconazole, micafungin) u. Normal saline 10 -15 m. L/kg prior to each dose u. Consider longer infusion times u. Appropriate monitoring (Scr, serum electrolytes) u Management: u. Discontinuation of amphotericin B and substitution with alternative antifungal therapy if possible u. Kidney injury may be reversible or irreversible after discontinuation 22

Radiographic Contrast Media u. Contrast media-induced nephrotoxicity (CIN) can occur in up to 50% of patients with pre-existing CKD or diabetes mellitus u. Nephrotoxicity results from acute renal ischemia and direct cellular toxicity due to increased exposure to contrast media following reduced blood flow u. Kidney injury may be irreversible, especially in those with pre-existing kidney disease 23

Radiographic Contrast Media u Clinical Presentation: u↑ Scr, BUN u. Non-oliguric or irreversible oliguria (urine < 500 m. L/d) in highrisk patients ugranular casts on urinalysis (not always) u. Fractional excretion of sodium <1% u Risk Factors: CKD (GFR <60 m. L/min), volume depletion, heart failure, hypotension, diabetic nephropathy, large volumes/doses, concurrent nephrotoxic drugs 24

Radiographic Contrast Media u Prevention: u. Use alternative diagnostic procedures if possible u. Avoid volume depletion and nephrotoxic drugs (i. e. NSAIDs) u. Use lowest volumes of contrast agents possible u. Volume expansion – normal saline prior to and continued for several hours after contrast exposure u. Oral N-acetylcysteine given prior to and following exposure u. Management: u. Supportive (monitoring, renal replacement therapy if irreversible damage occurs) 25

Acute/Allergic Interstitial Nephritis (AIN) u It consists of an acute idiosyncratic reaction involving inflammatory infiltration and edema of the intersititium u Signs of renal injury include oliguria, sterile pyuria, eosinophiluria (frequently absent) u Systemic signs and symptoms include fever, rash, arthralgia and eosinophilia u. More common in antibiotic-associated AIN than NSAID-associated u AIN is a hypersensitivity reaction and is expected to recur with re-challenge 26

β-lactams (including cephalosporins) & NSAIDs u Mechanism: Allergic hypersensitivity response via an antibody- or cellmediated (commonly a T-cell interstitial infiltrate) immune mechanism u Clinical Presentation: uβ-lactams – Average onset of 2 weeks from initiation u. Fever (27 -80%), maculopapular rash (15 -25%), eosinophilia (2380%) arthralgia (45%), oliguria (50%) u. NSAIDs – Average onset of 6 months from initiation u. Fever, rash, and eosinophilia occur in <10% while nephrotic syndrome (proteinuria >3. 5 g/d) occurs in >70% or patients u Risk Factors: None identified 27

β-lactams (including cephalosporins) & NSAIDs u Prevention: u. No specific preventative measures u. Appropriate monitoring so that prompt discontinuation can improve the chances of complete renal recovery u Management: u. Discontinue offending drug u. High-dose oral prednisone u. Monitor renal function (Scr, BUN, etc. ) for signs of improvement u. Document the reaction to avoid re-exposure u. Kidney injury may be reversible or irreversible 28

Other drugs Associated with ATN and AIN Acute Tubular Necrosis Acute Interstitial Nephritis u Chemotherapy u Cisplatin, carboplatin, cytarabine, 5 -fluoruracil, ifosfamide, u Ciprofloxacin u Omeprazole, lansoprazole u Cimetidine, ranitidine u Loop diuretics u Allopurinol u Vancomycin u Sulfonamides u IVIG u Rifampin u 5 -aminosalicylates u Tenofovir, cidofovir, adefovir u Zoledronate 29

Crystal Nephropathy Postrenal Injury 30

Crystal Nephropathy u Direct Intratubular Obstruction & Nephrolithiasis uvia drug precipitation (crystallization) u. Volume depletion and the resulting production of concentrated, acidic urine can precipitate drugs unable to remain in solution at ↓ p. H u. Abnormal crystal precipitation in the renal collecting system leading to pain, hematuria, infection, or urinary tract obstruction u Indirect Intratubular Obstruction u. Drugs may indirectly produce large amounts of endogenous toxins (i. e. uric acid, myoglobin) leading to intratubular obstruction and direct cellular damage 31

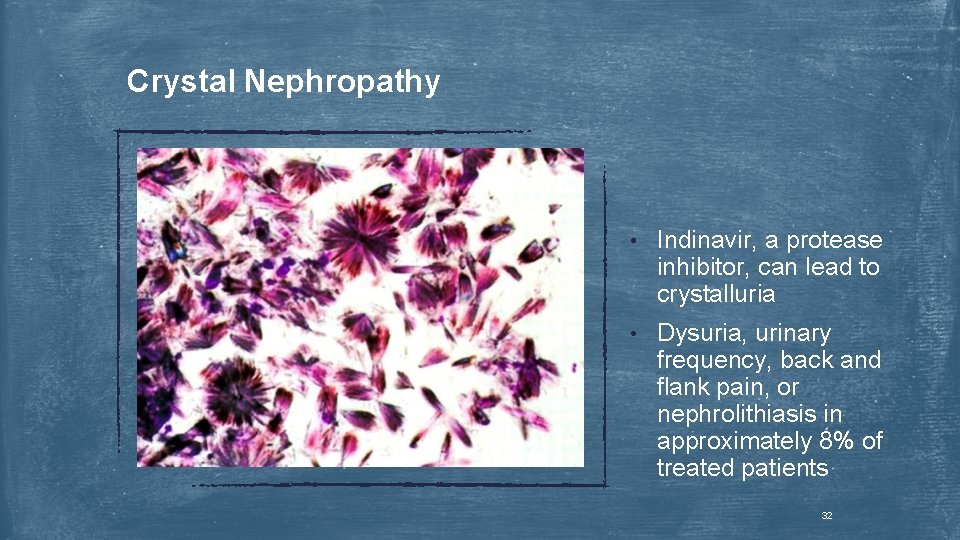
Crystal Nephropathy • Indinavir, a protease inhibitor, can lead to crystalluria • Dysuria, urinary frequency, back and flank pain, or nephrolithiasis in approximately 8% of treated patients 32

Direct Intratubular Obstruction & Nephrolithiasis u. Medications: u. Acyclovir u. Indinavir u. Tenofovir u. Atazanavir u. Methotrexate (IV) u. Sulfadiazine u. Triamterene u. Ciprofloxacin u. Mechanism: Insolubility of drug in either alkaline or acidic urine + low urine volume → precipitation of drug → crystalluria → obstruction of tubule u. Poor alkaline solubility: Indinavir u. Poor acidic solubility: Acyclovir, triamterene, sulfadiazine, methotrexate 33

Direct Intratubular Obstruction & Nephrolithiasis u Clinical Presentation: u. May have asymptomatic crystalluria u↓ urine output u↑ Scr, hematuria, pyuria, pain and crystalluria 34

Direct Intratubular Obstruction & Nephrolithiasis u Risk Factors: Volume depletion (fluid loss or sequestration) u Prevention: u. Hydration and prevention of volume depletion (crystal precipitation can be prevented in 75% of indinavir treated patients if they consume 2 -3 L of fluid per day) u. Urinary alkalinisation for drugs with poor acidic solubility u. Potassium citrate or sodium bicarbonate u Management: u. Discontinue drug (kidney injury is usually reversible) u. Volume resuscitation 35

Indirect Tubular Obstruction Tumor Lysis Syndrome Rhabdomyolysis u Antineoplastic u Statin-induced u Acute u Tubular u Treatment agents increase circulating by-products of tumor breakdown oliguric or anuric kidney injury is a result of uric acid crystal obstruction includes hydration, allopurinol and urinary alkalinisation rhabdomyolysis is rare (1 in 1000) but the risk is increased with drug interactions precipitation of myoglobin results in AKI and production of red-brown urine includes hydration/volume expansion and potentially, urinary alkalinisation 36