DRUG THERAPY OF KIDNEY DISEASES NEPHROLOGY Kidney disease





































- Slides: 46

DRUG THERAPY OF KIDNEY DISEASES

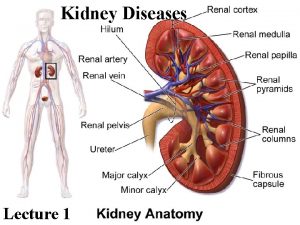
NEPHROLOGY Kidney disease and kidney failure are increasingly common in this 21 st century. Often caused by other conditions such as diabetes and hypertension, the severity of kidney disease can be greatly reduced if appropriate and early treatment. Diabetic nephropathy Hypertension Kidney stones Vasculitis Nephritis, glomerular and interstitial Chronic renal insufficiency, anemia and other complications Dialysis care Transplantation Edema and disorders of electrolytes

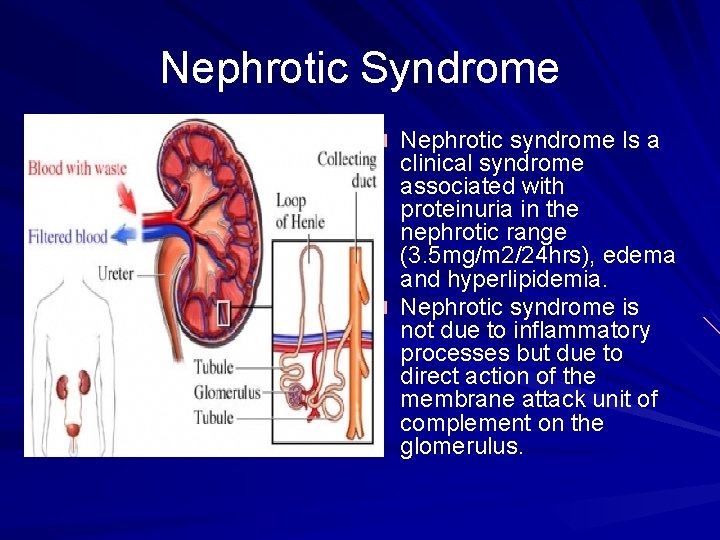
Nephrotic Syndrome Nephrotic syndrome Is a clinical syndrome associated with proteinuria in the nephrotic range (3. 5 mg/m 2/24 hrs), edema and hyperlipidemia. Nephrotic syndrome is not due to inflammatory processes but due to direct action of the membrane attack unit of complement on the glomerulus.

Clinical suspicion It should be suspected when a patient presents with generalized oedema, protein detected in urine, hypoalbuminemia.

Some of the symptoms of nephrotic syndrome swelling, especially around the eyes, ankles, and feet. Other symptoms include weight gain, because of the fluid and swelling, loss of appetite, and vomiting. serious side effects such as pleural effusion, high blood pressure, and problems with the liver.

Diagnosis of nephrotic syndrome The diagnosis maybe established with proteinuria in the nephrotic range alone without the other criteria. 1. Proteinuria > 3. 5 g/m 2/day: On detection of protein in urine a 24 hour urinary protein test is in order. There are two cut off values for this test: 1. increased urinary protein <1. 5 g/day; this is refered to as isolated proteinuria. 2. >3. 5 g/day; this establishes nephrotic syndrome. 2. Hypoalbuminaemia 3. Hyperlipidaemia: As albumen in serum is lost through the kidneys, the liver increases its production of albumen and concomitantly increases the production of cholesterol. 4. Oedema: resulting from loss of intravascular fluid to the extravascular space due decreased intravascular oncotic pressure (decreased albumen). However, the presence of proteinuria in the nephrotic range (3. 5 g/day) establishes the diagnosis. Differential diagnosis: proteinuria

Diagnosis of nephrotic syndrome Nitrogen balance diorders, hypercoaguloability, disturbances of calcium and bone metabolism, and thyroid hormones are often found in NS.

Several different causes have been identified for nephrotic syndrome. Damage to the small vessels, or glomeruli, can usually be traced to one of the following conditions: � Minimal change disease. This is the most common cause of this disorder in children. In children it results in abnormal kidney function, but is often deceiving because tissue samples look normal or nearly normal under a microscope. When this disorder is the cause of nephrotic syndrome, doctors are often unable to discern its cause. � Focal segmental glomerulosclerosis. When this disorder occurs, the glomeruli are scarred, which prevents them from working efficiently. The scarring might be caused by genetic factors, the presence of another disease, or for no discernible reason.

Membranous nephropathy. In this disorder, the membranes inside the glomeruli thicken, making it difficult for them to filter properly. This thickening is thought to be caused by several different problems, including hepatitis B, malaria, lupus, and cancer. � Systemic lupus erythematosus. This is a chronic inflammatory disease, and can lead to serious damage of the kidneys. � Diabetic kidney disease. Diabetic nephropathy, or kidney damage, is particularly common in poorly controlled diabetes or in people who have high blood pressure. � Amyloidosis. This is a disorder that occurs when amyloid proteins accumulate in the organs. This build-up damages the kidneys’ filtering system.

Nephrotic syndrome can be caused by primary and secondary Glomerulonephritis A. Primary Glomerulonephritis GN with minimal lessions Glomerulosclerotic focal Membranous Glomerulonephritis GN membranoproliferatif Other proliferative B. Secondary Glomerulonephritis 1. Infections: HIV, Hepatitis B and C virus Syphilis, Malaria TBC, Leprosy 2. Malignancy: Adenosarcoma Lymphoma Multiple Myeloma Renal Carcinoma

3. Connective Tissue Disease: SLE Rhematoid Arthritis Mixed Cinnective Tissue Disease 4. Drugs and Toxins: NSIDs Gold Preparations Penicillamine Probenecid Mercury Captopril Heroin 5. Other: Diabetes Mellitus Amyloidosis Pre-Eclampsia Vesikoureter reflux

Treatment of nephrotic syndrome depends primarily on the cause, however, it frequently involves the use the glucorticoids given over long periods of time. Especially in cases of minimal change disease. Here the role of steroids is to suppress the autoimmune basis for this disease. The use of cytotoxic agents maybe required in some cases (e. g. cyclophosphamide). Dietary salt control, treatment of hypertension and hypercholestrolemia is also recommended. ACE inhibitors, in addition to controlling blood pressure have also been found to decrease the protein loss. Diuretics may help control the edema and the hypertension.

Chronic Renal Failure Chronic renal failure (CRF) is a slowly progressive loss of renal function over a period of months or years. The kidneys attempt to compensate for renal damage by hyper filtration with the remaining functional nephrons. Chronic loss of function causes generalized wasting or shrinking and progressive scarring within all parts of the kidneys. In time, overall scarring obscures the site of the initial damage. But, it is not until over 70% of the normal combined function of both kidneys is lost that most patients begin to experience symptoms of kidney failure.

Causes and Symptoms of CRF: • Diabetes • General ill feeling and fatigue • High Blood Pressure • Generalized itching & dry skin • Glomerulonephritis • Headaches • Polycycstic Kidney Disease • Weight loss • Analgesic Nephropathy • Appetite Loss • Polycycstic Kidney Disease


Chronic Renal Failure A. Definitions 1. Azotemia - elevated blood urea nitrogen (BUN >28 mg/d. L) and creatinine (Cr>1. 5 mg/d. L) 2. Uremia - azotemia with symptoms or signs of renal failure 3. End Stage Renal Disease (ESRD) - uremia requiring transplantation or dialysis 4. Chronic Renal Failure (CRF) - irreversible kidney dysfunction with azotemia >3 months 5. Creatinine Clearance (CCr) - the rate of filtration of creatinine by the kidney (GFR marker) 6. Glomerular Filtration Rate (GFR) - the total rate of filtration of blood by the kidney


B. Etiology 1. Episodes of ARF (usually acute tubular necrosis) often lead, eventually, to CRF 2. Over time, combinations of acute renal insults are additive and lead to CRF 3. The definition of CRF requires that at least 3 months of renal failure have occurred 4. Causes of Acute Renal Failure (ARF) a. Prerenal azotemia - renal hypoperfusion, usually with acute tubular necrosis b. Intrinsic Renal Disease, usually glomerular disease c. Postrenal azotemia - obstruction of some type


1. Common Underlying Causes of CRF There about 50, 000 cases of ESRD per year Diabetes: most common cause ESRD (risk 13 x ) Over 30% cases ESRD are primarily to diabetes CRF associated HTN causes ~ 23% ESRD cases Glomerulonephritis accounts for ~10% cases Polycystic Kidney Disease - about 5% of cases Rapidly progressive glomerulonephritis (vasculitis) - about 2% of cases h. Renal (glomerular) deposition diseases i. Renal Vascular Disease - renal artery stenosis, atherosclerotic vs. fibromuscular a. b. c. d. e. f. g.

a. Medications - especially causing tubulointerstitial diseases (common ARF, rare CRF) b. Analgesic Nephropathy over many years c. Pregnancy - high incidence of increased creatinine and HTN during pregnancy in CRF Black men have a 3. 5 -4 fold increased risk of CRF compared with white men a. Blood pressure and socioecomonic status correlated with CRF in whites and blacks b. Unclear if blacks have increased risks when blood pressure and income are similar

Analgesic Nephropathy a. Slow progression of disease due to chronic daily ingestion of analgesics b. Drugs associated with this entity usually contain two antipyretic agents and either caffeine or codeine c. More common in Europe and Australia than USA d. Polyuria is most common early symptom e. Macroscopic hematuria / papillary necrosis f. Chronic interstitial nephritis, renal papillary necrosis, renal calcifications g. Associated with long-term use of non-steroid anti-inflammatory drugs

Analgesic Nephropathy cont’d Patients at risk: DM, CHF, CRI, Hepatic disease, elderly, etc Pathophysiologynonselective NSAIDS inhibit synthesis vasodilatory prostaglandin in the kidney=prerenal state ARF COX 2 not so innocent afterall.

Electrolyte Abnormalities 1. Excretion of Na+ is initially increased, probably due to natriuretic factors 2. As glomerular filtration rate (GFR) falls, Fe. Na rises a. Maintain volume until GFR <10 -20 m. L/min, then edema b. Renal failure with nephrotic syndrome, early edema c. Cannot conserve Na+ when GFR <25 m. L/min, and Fe. Na rises with falling GFR 3. Tubular K+ secretion is decreased a. Aldosterone mediated. Also increased fecal loss of K+ (up to 50% of K ingested) b. Cannot handle bolus K+, avoid drugs high K+ c. Do not use K+ sparing diuretics

Control of acids Normally, produce ~1 m. Eq/kg/day H+ When GFR <40 m. L/min then decrease NH 4+ excretion adds to metabolic acidosis When GFR <30 m. L/min then urinary phosphate buffers decline and acidosis worsens Bone Ca. CO 3 begins to act as the buffer and bone lesions result (renal osteodystrophy) Usually will not have wide anion gap even with acidosis if can make urine Acidosis caused by combination hyperchloremia and hypersulfatemia Defect in renal generation of HCO 3 -, as well as retention of nonvolatile acids

Loss of urine diluting and concentrating abilities a. Osmotic diuresis due to high solute concentration for each functioning nephron b. Reduce urinary output only by reducing solute excretion c. Major solutes are salt and protein, so these should be decreased

Bone Metabolism ↓GFR leads to ↑ phosphate ↓ calcium + acidosis In addition, ↑ tubular resorption Ca+ ↑ hypocalcemia Other defects include acidosis and decreased dihydroxy-vitamin D production Bone acts as a buffer for acidosis, leading to chronic bone loss in renal failure Low vitamin D causes poor calcium absorbtion and hyperparathyroidism (high PTH) Increased PTH maintains normal serum Ca 2+ and PO 42 - until GFR <30 m. L/min Chronic hyperparathyroidism and bone buffering of acids leads to severe osteoporosis

7. Other abnormalities a. Slight hypermagnesemia with inability to excrete high magnesium loads b. Uric acid retention occurs with GFR <40 m. L/min c. Vitamin D conversion to dihydroxy-Vitamin D is severely decreased d. Erythropoietin (EPO) levels fall and anemia develops 8. Accumulation of normally excreted substances, "uremic toxins", MW 300 -5000 daltons

Uremic Syndrome 1. Symptomatic azotemia 2. Fever, Malaise 3. Anorexia, Nausea 4. Mild neural dysfunction 5. Uremic pruritus

Associated Problems and Treatment Immunosuppression a. Patients with CRF, even pre-dialysis, are at increased risk for infection b. Cell mediated immunity is particularly impaired c. Hemodialysis seems to increase immunocompromise d. Complement system is activated during hemodialysis e. Patients with CRF should be vaccinated aggressively

Anemia a. Due to reduced erythropoietin production by kidney b. Occurs when creatinine rises to >2. 5 -3 mg/d. L c. Anemia management: Hct goal - 33% Hyperphosphatemia a. Decreased excretion by kidney b. Increased phosphate load from bone metabolism (by high parathyroid hormone levels) c. Increased PTH levels leads to renal bone disease d. Eventually, parathyroid gland hyperplasia occurs e. Danger of calciphylaxis (Ca x Phosp product)

Hypertension a. Blood pressure control is very important to slowing progression of renal failure b. About 30% of end-stage renal disease (ESRD) is related to hypertension c. Overall risk of CRF with creatinine >2. 0 mg/d. L is ~2 X in five years with HTN d. Patients with grade IV (severe) HTN have 22 X increased risk vs. normal for CRF e. Targetted mean pressure 92 -98 mm Hg in patients with renal failure and proteinuria f. Patients with HTN and albuminuria >1 gm/day, blacks, diabetics have higher ESRD risk

g. ACE inhibitors shown be most effective at preserving renal function by preferential dilation efferent arterioles which IGCP. h. ACE inhibitors are avoided in patients with serum creatinine >2. 5 -3 mg/d. L Goal B/P 130/80 mm. Hg for all renal patients. African American study of kidney disease (AASK), ACE >>BB or CCB Heart Outcome Prevention and evaluation study (HOPE), ramipril dec mobidity/mortality. Less hyperkalemia with ARB vs ACE.

Poor coagulation a. Platelet dysfunction - usually with prolonged bleeding times b. May be partially reversed with DDAVP administration 7. Proteinuria >0. 25 gm per day is an independent risk factor for renal decline] 8. Uremic pruritus may respond to dialysis or opiate antagonists (eg. naltrexone 50 mg/d)

F. Evaluation 1. Search for underlying causes 2. Laboratory a. Full Electrolyte Panel b. Calcium, phosphate, uric acid, magnesium and albumin c. Urinalysis, microscopic exam, quantitation of protein in urine (protein: creatinine ratio) d. Calculation of creatinine clearance and protein losses e. Complete blood count f. Consider complement levels, protein electrophoresis, antinuclear antibodies, ANCA g. Renal biopsy - particularly in mixed or idiopathic disease

3. Radiographic Evaluation a. Renal Ultrasound - evaluate for obstruction, stones, tumor, kidney size, chronic change b. Duplex ultrasound or angiography or spiral CT scan to evaluate renal artery stenosis c. MRA preferred over contrast agents 4. Bone Evaluation a. Severe secondary hyperparathyroidism can lead to osteoporosis b. Some patients will require parathyroidectomy to help prevent this c. Bone densitometry should be done on patients with CRF

Treatment The most important differential diagnosis is to decide whether the renal failure is acute or chronic. History could provide indications as to the onset of problems from the history of urinary changes in terms of quantity and quality; and history of a loss in body weight, chronic fatigue etc. The presence of anaemia suggests CRF; bilateral small kidneys suggest CRF; neuropathy, lipiduria, and osteodystrophy. The presence of loin pain is always a good sign indicating that the kidney is still responsive. CRF cannot be treated apart from by renal transplant. In the period usually required to find a transplant, dialysis (renal function replacement therapy) is the only way to clear waste products from the blood that are usually excreted through the urine (urea, potassium). Replacement of erythropoietin and vitamin D 3, two hormones processed by the kidney, is usually necessary, as is calcium.

Pre-Dialysis Treatment 1. Maintain normal electrolytes a. Potassium, calcium, phosphate are major electrolytes affected in CRF b. ACE inhibitors may be acceptable in many patients with creatinine >3. 0 mg/d. L c. ACE inhibitors may slow the progression of diabetic and non-diabetic renal disease d. Reduce or discontinue other renal toxins (including NSAIDS) e. Diuretics (eg. furosemide) may help maintain potassium in normal range f. Renal diet including high calcium and low phosphate

Reduce protein intake to <0. 6 gm/kg body weight a. Appears to slow progression of diabetic and nondiabetic kidney disease b. In type 1 diabetes mellitus, protein restriction reduced levels of albuminuria c. Low protein diet did not slow progression in children with CRF Underlying Disease a. Diabetic nephropathy should be treated with ACE inhibitors until creatinine >2. 5 -3 mg/d. L b. Hypertension should be aggressively treated (ACE inhibitors are preferred)

a. Caution with use of ACE inhibitors in renal 1. a. b. c. d. e. artery stenosis Ramipril in Non-Diabetic Proteinuric Nephropathy Ramipril is a second generation ACE inhibitor with efficacy in HTN and heart Failure In patients with non-diabetic proteinuria >3 gm/day, ramipril reduced progression Drug was titrated to a diastolic BP under 90 mm. Hg Ramipril reduced rate of GFR decline by >20%, more than anti-hypertensive drugs alone Data for patients with <3 gm/day proteinuria is still being evaluated

a. Ramipril may be preferred agent for treatment of non b. a. b. c. d. e. f. -diabetic proteinuric nerphropathy A meta-analysis of ACE inhibitors in non-diabetic renal disease showed benefit H. Hemodialysis Indications Uremia - azotemia with symptoms and/or signs Severe Hyperkalemia Volume Overload - usually with congestive heart failure (pulmonary edema) Toxin Removal - ethylene glycol poisoning, theophylline overdose, etc. An arterio-venous fistula in the arm is created surgically Catheters are inserted into the fistula for blood flow to dialysis machine

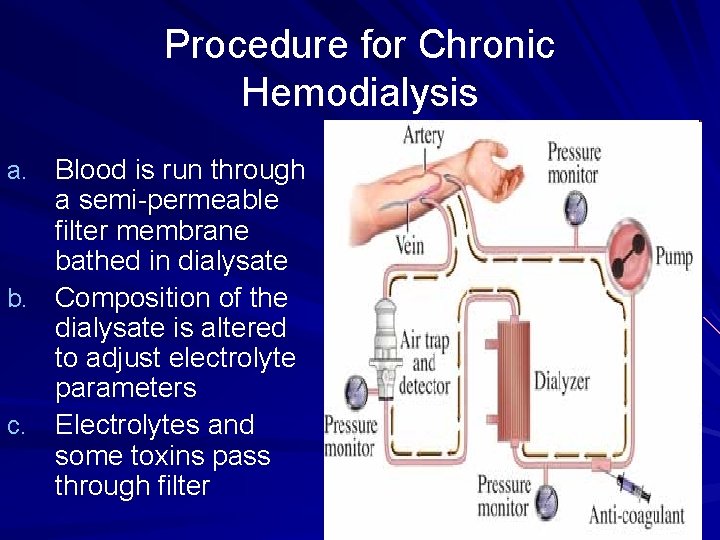
Procedure for Chronic Hemodialysis a. Blood is run through a semi-permeable filter membrane bathed in dialysate b. Composition of the dialysate is altered to adjust electrolyte parameters c. Electrolytes and some toxins pass through filter

Hemodialysis machine

Hemodialysis By controlling flow rates (pressures), patient's intravascular volume can be reduced Most chronic hemodialysis patients receive 3 hours dialysis 3 days per week

a. b. c. d. e. f. Efficacy Some acids, BUN and creatinine are reduced Phosphate is dialyzed, but quickly released from bone Very effective at reducing intravascular volume/potassium Once dialysis is initiated, kidney function is often reduced Not all uremic toxins are removed and patients generally do not feel "normal" Response of anemia to erythropoietin is often suboptimal with hemodialysis

Chronic Hemodialysis Medications a. Anti-hypertensives - labetolol, CCB, ACE inhibitors b. Erythropoietin (Epogen®) for anemia in ~80% dialysis pts c. Vitamin D Analogs - calcitriol given intravenously d. Calcium carbonate or acetate to phosphate and PTH e. Rena. Gel, a non-adsorbed phosphate binder, is being developed for hyperphosphatemia f. DDAVP may be effective for patients with symptomatic platelet problems
 Choronic kidney disease
Choronic kidney disease Low potassium diet nemo
Low potassium diet nemo Albumin kidney disease
Albumin kidney disease Carlee oakley
Carlee oakley Albumin kidney disease
Albumin kidney disease Kara potter
Kara potter Symptomatic polycystic kidney disease
Symptomatic polycystic kidney disease Chronic kidney disease near atwater
Chronic kidney disease near atwater Miami pediatric nephrology seminar
Miami pediatric nephrology seminar Eugene springfield nephrology associates
Eugene springfield nephrology associates Coffin shaped kidney stones
Coffin shaped kidney stones Obstractive uropathy
Obstractive uropathy Nephrology nursing certification commission
Nephrology nursing certification commission Nephrology case presentation
Nephrology case presentation Pinehurst nephrology associates
Pinehurst nephrology associates Caribbean institute of nephrology
Caribbean institute of nephrology Deliberate adulteration examples
Deliberate adulteration examples Drug therapy problems
Drug therapy problems Drug therapy management
Drug therapy management Nursing process in drug therapy
Nursing process in drug therapy Bharathi viswanathan
Bharathi viswanathan Triple therapy for peptic ulcer disease
Triple therapy for peptic ulcer disease Gene therapy for sickle cell disease
Gene therapy for sickle cell disease Peptic ulcer anatomy
Peptic ulcer anatomy Pud triple therapy
Pud triple therapy Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Psychodynamic and humanistic therapies have in common
Psychodynamic and humanistic therapies have in common Bioness integrated therapy system price
Bioness integrated therapy system price Diseases spread by columbian exchange
Diseases spread by columbian exchange Mastigophora diseases
Mastigophora diseases Waterwashed diseases
Waterwashed diseases Introduction of nutrients
Introduction of nutrients Perianal pruritus
Perianal pruritus Chapter 24 sexually transmitted diseases and hiv/aids
Chapter 24 sexually transmitted diseases and hiv/aids Chapter 21 mental health diseases and disorders
Chapter 21 mental health diseases and disorders Purulent diseases of lungs and pleura
Purulent diseases of lungs and pleura Is athlete's foot communicable or noncommunicable
Is athlete's foot communicable or noncommunicable Diseases caused by dust
Diseases caused by dust Protein deficiency diseases
Protein deficiency diseases Nursing management of reproductive tract infection
Nursing management of reproductive tract infection King of diseases
King of diseases Diseases spread by columbian exchange
Diseases spread by columbian exchange An exogenous disease originates outside the body.
An exogenous disease originates outside the body. 10 diseases of lymphatic system
10 diseases of lymphatic system Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Vitamin deficiency diseases chart
Vitamin deficiency diseases chart Non communicable diseases infographic
Non communicable diseases infographic