DRUG POLICY OF THE EUROPEAN UNION SILVIO GARATTINI



























- Slides: 49

DRUG POLICY OF THE EUROPEAN UNION SILVIO GARATTINI SIVIGLIA 15 May 2007

BIAS IN CLINICAL TRIALS • EUROPEAN LEGISLATION

Institutional location Industry, consumers or public health?


Quality, efficacy, safety Necessary, not always sufficient

CONFIDENTIALITY • IS JUSTIFIED? • MINORITY POSITION • REASONS FOR WITHDRAWAL • RESULTS OF COMMITTMENTS

ADDED VALUE • TOO MANY TRIALS USE PLACEBO OR NO-CONTROLS

ADDED VALUE • TOO MANY TRIALS USE PLACEBO OR NO-CONTROLS • WHEN COMPARISONS ARE MADE DESIGNS OF EQUIVALENCE OR NON INFERIORITY ARE

ADDED VALUE • TOO MANY TRIALS USE PLACEBO OR NO-CONTROLS • WHEN COMPARISONS ARE MADE DESIGNS OF EQUIVALENCE OR NON INFERIORITY ARE • SELECTION OF COMPARATOR IS FREQUENTLY INADEQUATE

ADDED VALUE • TOO MANY TRIALS USE PLACEBO OR NO-CONTROLS • WHEN COMPARISONS ARE MADE DESIGNS OF EQUIVALENCE OR NON INFERIORITY ARE • SELECTION OF COMPARATOR IS FREQUENTLY INADEQUATE • FOCUS ON SELECTED ADVERSE REACTIONS

ADDED VALUE • TOO MANY TRIALS USE PLACEBO OR NO-CONTROLS • WHEN COMPARISONS ARE MADE DESIGNS OF EQUIVALENCE OR NON INFERIORITY ARE • SELECTION OF COMPARATOR IS FREQUENTLY INADEQUATE • FOCUS ON SELECTED ADVERSE REACTIONS • SMALL ADVANTAGES FOR NIGH PRICES


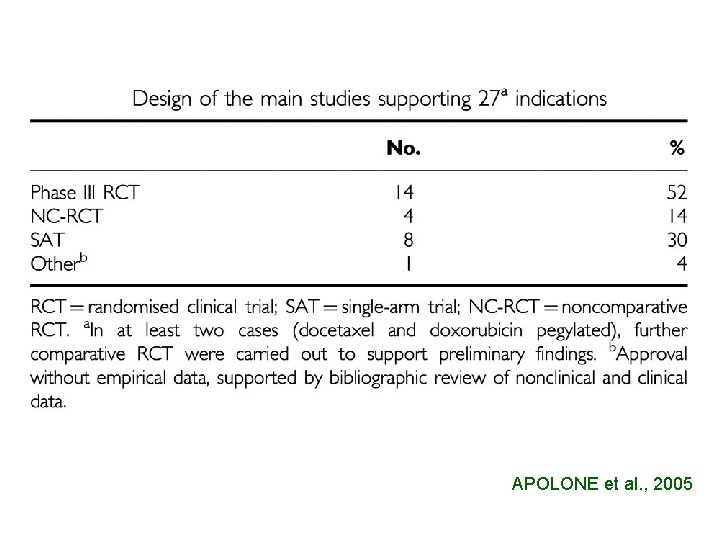
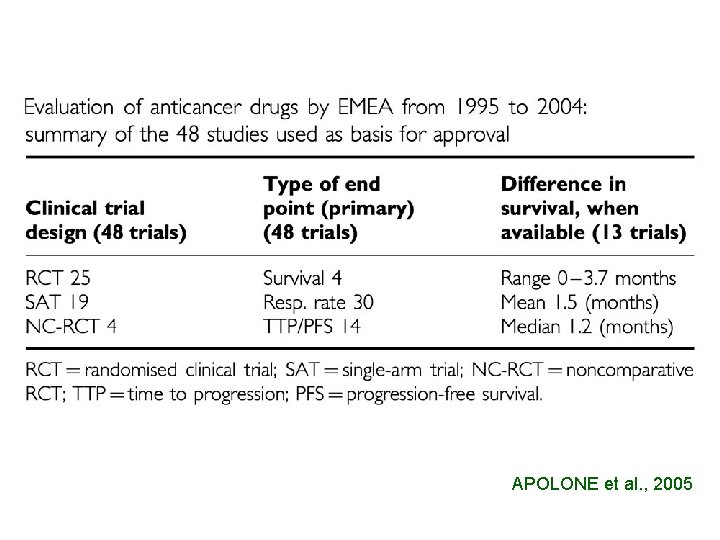
18 ANTICANCER AGENTS 21 INDICATIONS 12 9 ONLY PHASE III 6 EQUIVALENCE OR NON INFERIORITY 3 SUPERIORITY

APOLONE et al. , 2005

APOLONE et al. , 2005

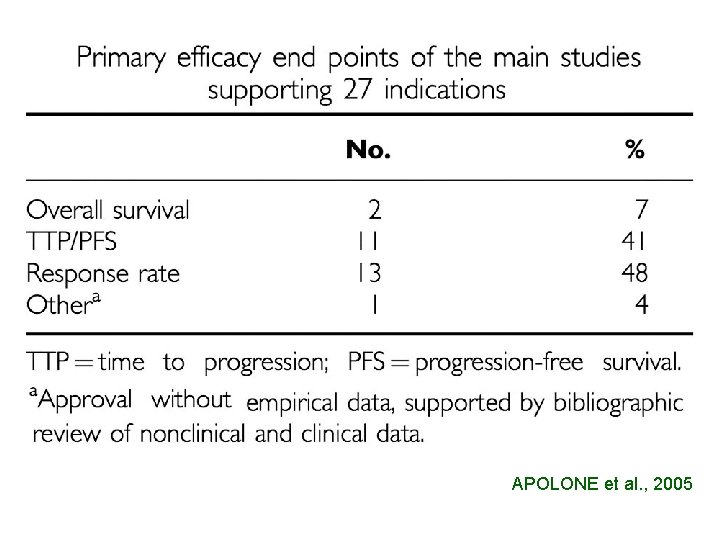
APOLONE et al. , 2005

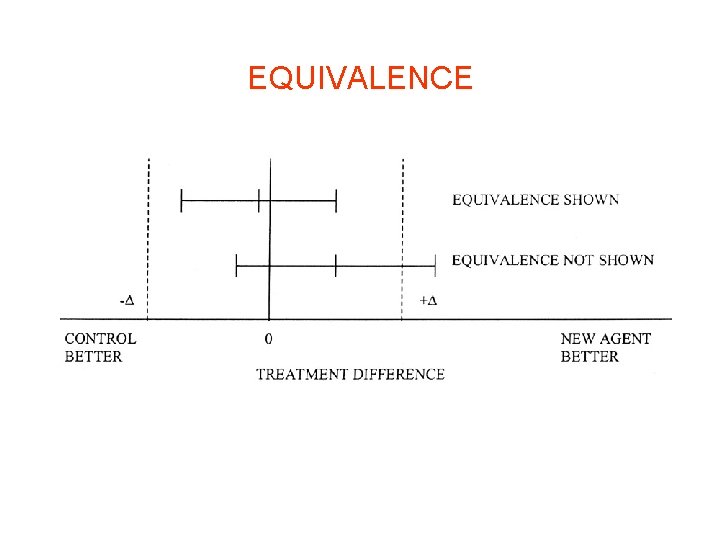
EQUIVALENCE


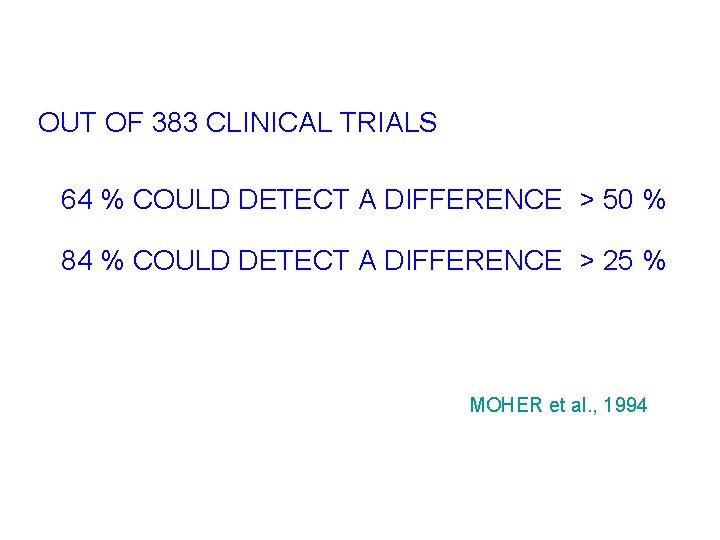
OUT OF 383 CLINICAL TRIALS 64 % COULD DETECT A DIFFERENCE > 50 % 84 % COULD DETECT A DIFFERENCE > 25 % MOHER et al. , 1994

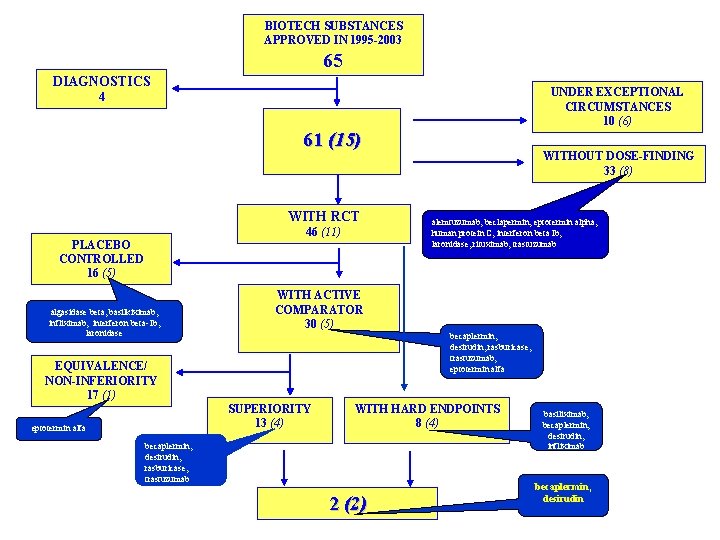
BIOTECH SUBSTANCES APPROVED IN 1995 -2003 65 DIAGNOSTICS UNDER EXCEPTIONAL CIRCUMSTANCES 10 (6) 4 61 (15) WITH RCT 46 (11) PLACEBO CONTROLLED 16 (5) algasidase beta, basilkiximab, infliximab, interferon beta-1 b, laronidase alemtuzumab, beclapermin, eptotermin alpha, human protein C, interferon beta Ib, laronidase, rituximab, trastuzumab WITH ACTIVE COMPARATOR 30 (5) becaplermin, desirudin, rasburicase, trastuzumab, eptotermin alfa EQUIVALENCE/ NON-INFERIORITY 17 (1) SUPERIORITY 13 (4) eptotermin alfa WITHOUT DOSE-FINDING 33 (8) WITH HARD ENDPOINTS 8 (4) becaplermin, desirudin, rasburicase, trastuzumab 2 (2) basiliximab, becaplermin, desirudin, infliximab becaplermin, desirudin

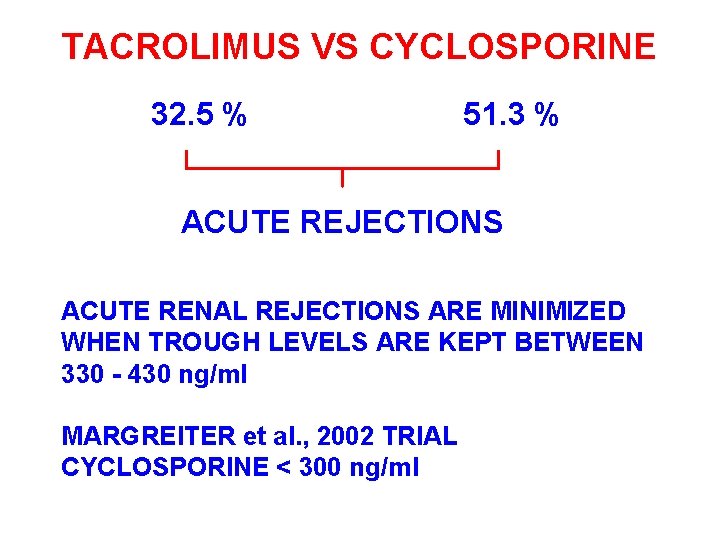
TACROLIMUS VS CYCLOSPORINE 32. 5 % 51. 3 % ACUTE REJECTIONS ACUTE RENAL REJECTIONS ARE MINIMIZED WHEN TROUGH LEVELS ARE KEPT BETWEEN 330 - 430 ng/ml MARGREITER et al. , 2002 TRIAL CYCLOSPORINE < 300 ng/ml

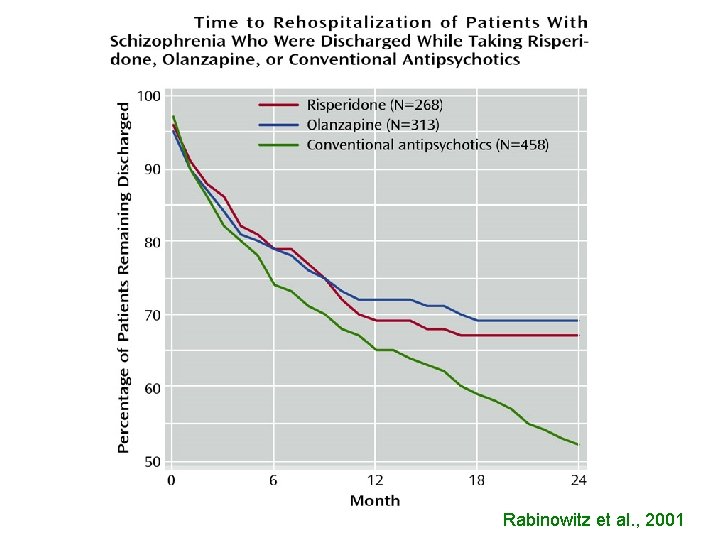
Rabinowitz et al. , 2001

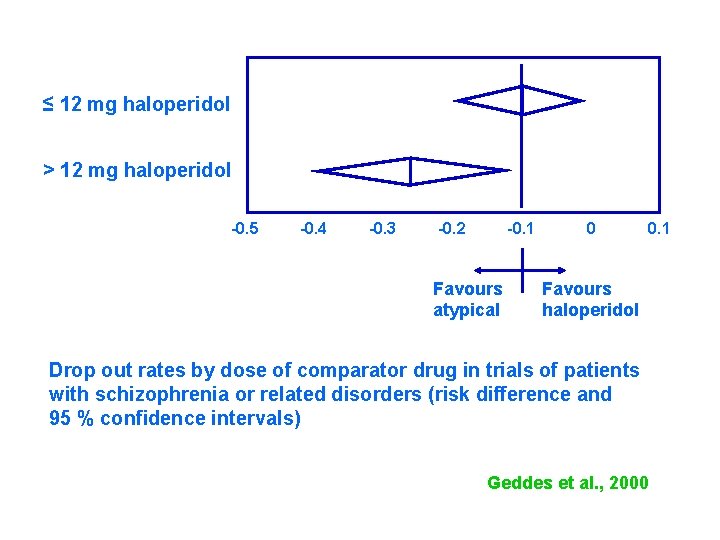
≤ 12 mg haloperidol > 12 mg haloperidol -0. 5 -0. 4 -0. 3 -0. 2 -0. 1 Favours atypical 0 0. 1 Favours haloperidol Drop out rates by dose of comparator drug in trials of patients with schizophrenia or related disorders (risk difference and 95 % confidence intervals) Geddes et al. , 2000

ATYPICAL ANTIPSYCHOTICS THE REDUCED EFFECT ON EPS IS THE FOCUS OF ADVERTISEMENT WEIGHT GAIN AND PROPENSITY TO DIABETES ?

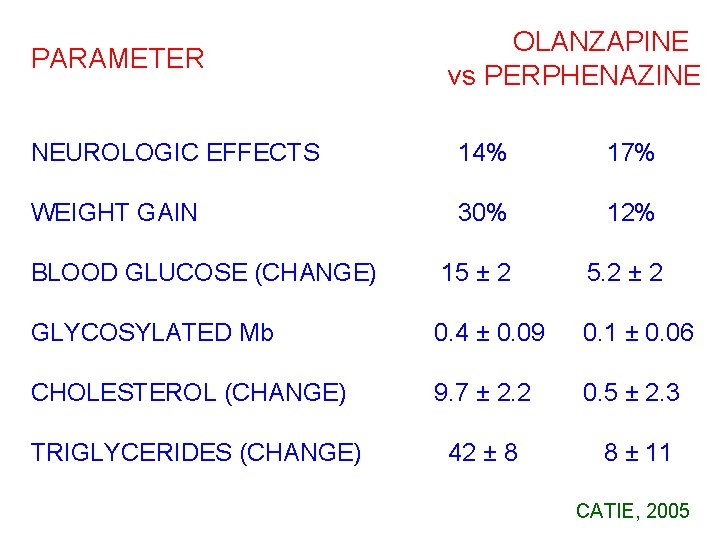
PARAMETER OLANZAPINE vs PERPHENAZINE NEUROLOGIC EFFECTS 14% 17%0 WEIGHT GAIN 30% 12%0 15 ± 20 5. 2 ± 200 00. 4 ± 0. 09 0. 1 ± 0. 06 9. 7 ± 2. 2 0. 5 ± 2. 30 42 ± 8 8 ± 11 BLOOD GLUCOSE (CHANGE) GLYCOSYLATED Mb CHOLESTEROL (CHANGE) TRIGLYCERIDES (CHANGE) CATIE, 2005

IF 4 MILLIONS SCHIZOPHRENIC PEOPLE WERE TREATED WITH ANTIPSYCHOTIC AGENTS CONFERRING A MEAN WEIGHT GAIN OF 4 Kg OVER A PERIOD OF 10 YEARS IN COMPARISON WITH A DRUG WITH LITTLE EFFECT ON BODY WEIGHT • ADDITIONAL 24, 560 DEATHS • ADDITIONAL 92, 720 CASES OF IMPAIRED GLUCOSE TOLERANCE • ADDITIONAL 120, 760 CASES OF HYPERTENSION FONTAINE et al. , 2001

Cumulative Incidence of the Primary End Point of a Confirmed Upper Gastrointestinal Event among All Randomized Patients. Vigor Study Group. N Engl J Med 2000

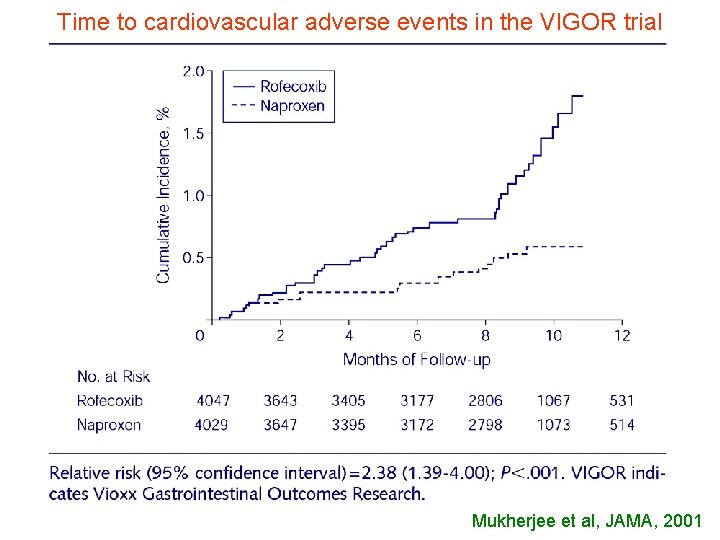
Time to cardiovascular adverse events in the VIGOR trial Mukherjee et al, JAMA, 2001

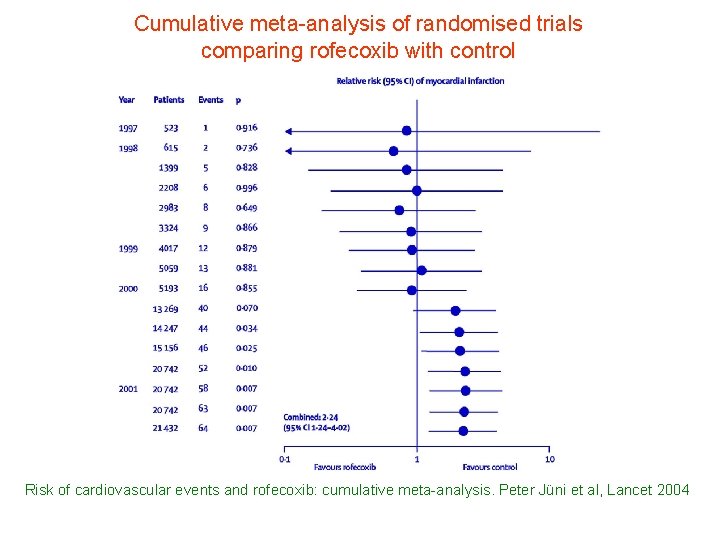
Cumulative meta-analysis of randomised trials comparing rofecoxib with control Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Peter Jüni et al, Lancet 2004

For newly licensed drugs, confidence about safety can only be provisional Rofecoxib • at the time of marketing approval (1999): safety data based on 5, 000 patients • at the time of withdrawal (2004): given daily to 2, 000 patients (sales in 2003 US $ 2. 5 billion)

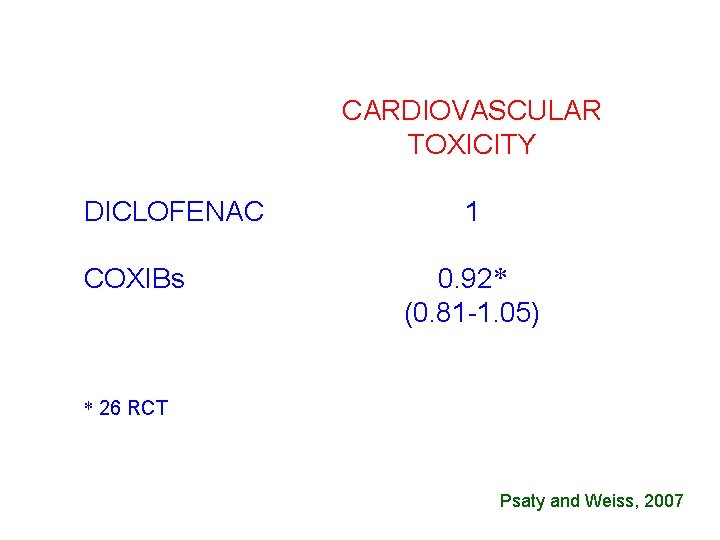
CARDIOVASCULAR TOXICITY DICLOFENAC COXIBs 1 0. 92* (0. 81 -1. 05) * 26 RCT Psaty and Weiss, 2007

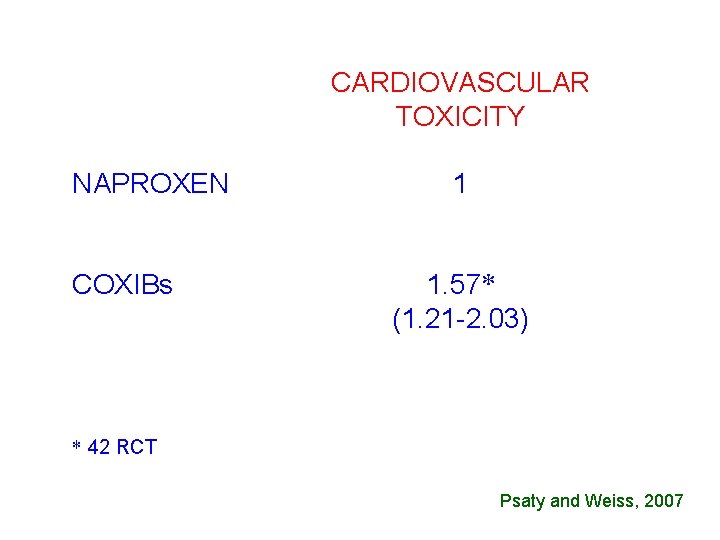
CARDIOVASCULAR TOXICITY NAPROXEN COXIBs 1 1. 57* (1. 21 -2. 03) * 42 RCT Psaty and Weiss, 2007

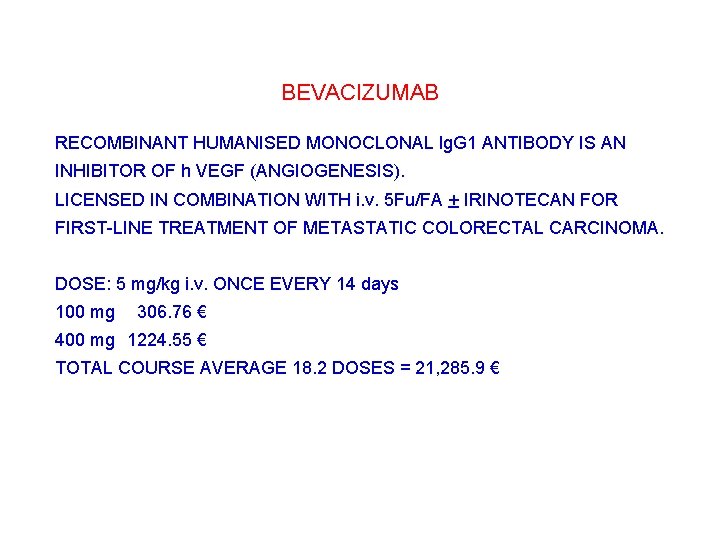
BEVACIZUMAB RECOMBINANT HUMANISED MONOCLONAL lg. G 1 ANTIBODY IS AN INHIBITOR OF h VEGF (ANGIOGENESIS). LICENSED IN COMBINATION WITH i. v. 5 Fu/FA + IRINOTECAN FOR FIRST-LINE TREATMENT OF METASTATIC COLORECTAL CARCINOMA. DOSE: 5 mg/kg i. v. ONCE EVERY 14 days 100 mg 0306. 76 € 400 mg 1224. 55 € TOTAL COURSE AVERAGE 18. 2 DOSES = 21, 285. 9 €

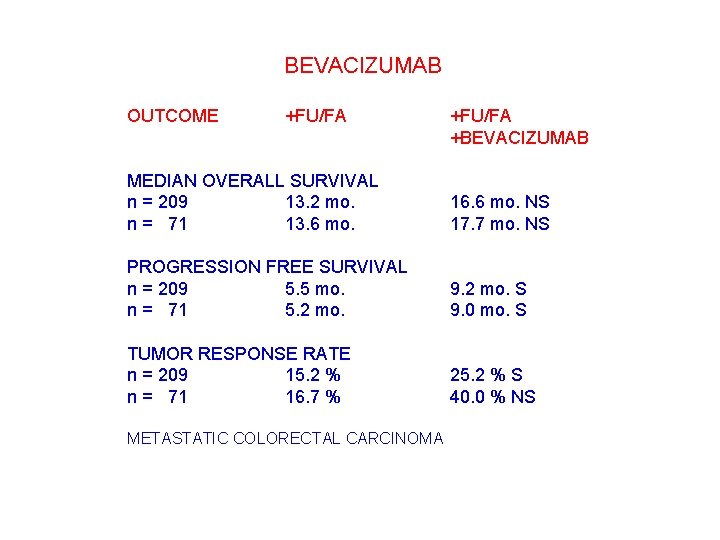
BEVACIZUMAB OUTCOME +FU/FA +BEVACIZUMAB MEDIAN OVERALL SURVIVAL n = 209 13. 2 mo. n = 071 13. 6 mo. 16. 6 mo. NS 17. 7 mo. NS PROGRESSION FREE SURVIVAL n = 209 5. 5 mo. n = 071 5. 2 mo. 9. 2 mo. S 9. 0 mo. S TUMOR RESPONSE RATE n = 209 15. 2 % n = 071 16. 7 % 25. 2 % S 40. 0 % NS METASTATIC COLORECTAL CARCINOMA

BEVACIZUMAB OUTCOME IFL- BEVACIZUMAB MEDIAN OVERALL SURVIVAL n=8 P 3 15. 6 mo. 20. 3 mo. PROGRESSION FREE SURVIVAL 06. 2 mo. 10. 6 mo. 34. 8 % 44. 8 % TUMOR RESPONSE RATE METASTATIC COLORECTAL CANCER

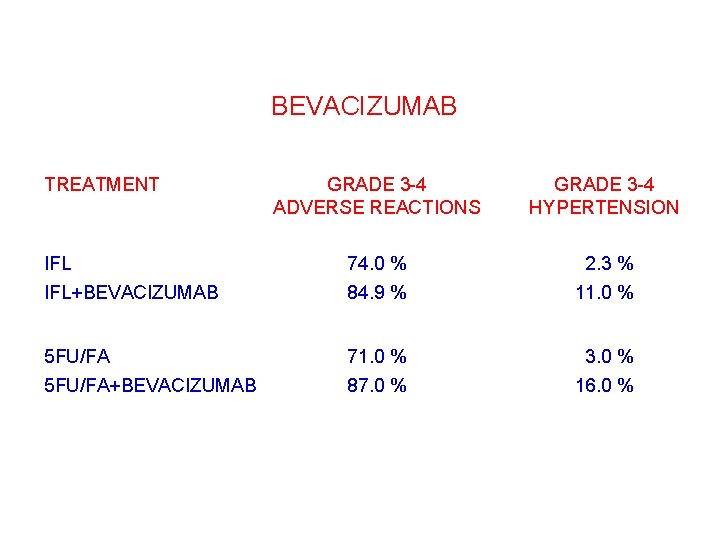
BEVACIZUMAB TREATMENT GRADE 3 -4 ADVERSE REACTIONS GRADE 3 -4 HYPERTENSION IFL+BEVACIZUMAB 74. 0 % 84. 9 % 02. 3 % 11. 0 % 5 FU/FA+BEVACIZUMAB 71. 0 % 87. 0 % 03. 0 % 16. 0 %

Paclitaxel-Carboplatin Alone or with Bevacizumab for Non-Small-Cell Lung Cancer Sandler et al. N Engl J Med 2006; 355: 2542 -50 Conclusions The addition of bevacizumab to paclitaxel plus carboplatin in the treatment of selected patients with non-small-cell lung cancer has a significant survival benefit with the risk of increased treatment-related deaths. (Clinical. Trials. gov number, NCT 00021060. )

Script 23 April 2007

PACLITAXEL-CARBOPLATIN ± BEVACIZUMAB Sandler et al. , NEJM 2006, 355, 2542 SURVIVAL BENEFIT 12 Pts MUST BE TREATED TO HAVE 1 MORE SURVIVAL AT 1 YEAR AT THE PRICE OF 1 TOXIC DEATH EVERY 24 PATIENTS TREATED MEDIAN OVERALL SURVIVAL 12. 3 mo. vs 10. 3 mo.

BEVACIZUMAB INCREASE OF SALES BY 76% IN 2006 vs 2005 WITH A TOTAL OF 2. 4 Billion $ SCRIPT, 29 th March 2007

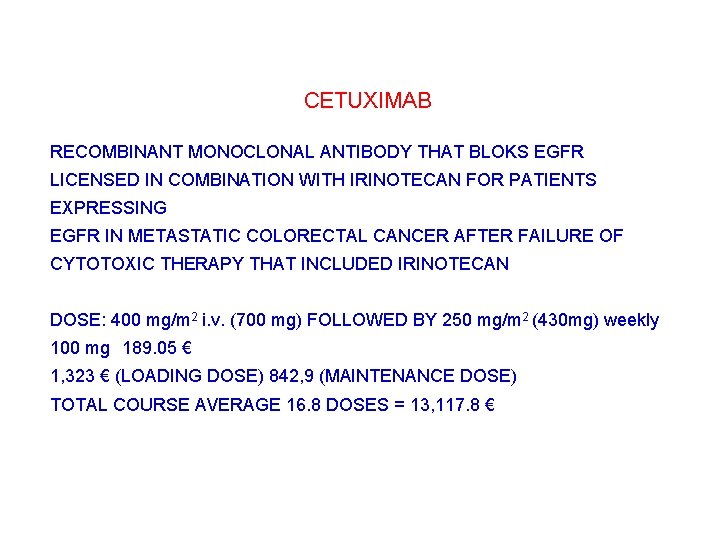
CETUXIMAB RECOMBINANT MONOCLONAL ANTIBODY THAT BLOKS EGFR LICENSED IN COMBINATION WITH IRINOTECAN FOR PATIENTS EXPRESSING EGFR IN METASTATIC COLORECTAL CANCER AFTER FAILURE OF CYTOTOXIC THERAPY THAT INCLUDED IRINOTECAN DOSE: 400 mg/m 2 i. v. (700 mg) FOLLOWED BY 250 mg/m 2 (430 mg) weekly 100 mg 189. 05 € 1, 323 € (LOADING DOSE) 842, 9 (MAINTENANCE DOSE) TOTAL COURSE AVERAGE 16. 8 DOSES = 13, 117. 8 €

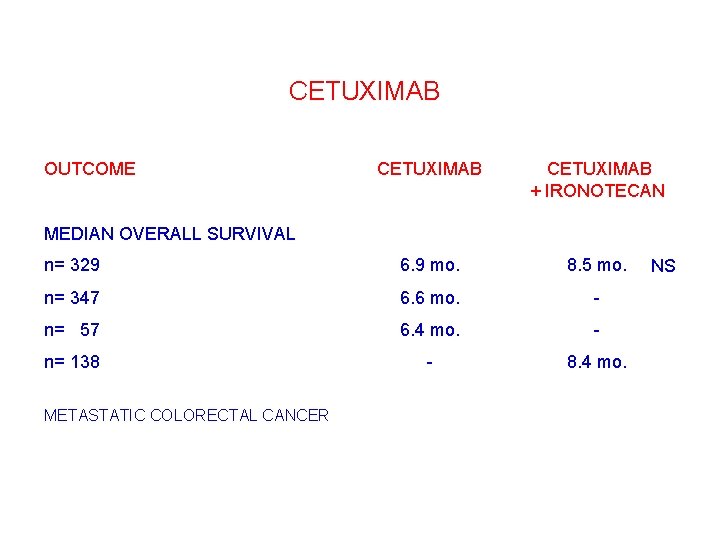
CETUXIMAB OUTCOME CETUXIMAB + IRONOTECAN n= 329 6. 9 mo. 8. 5 mo. n= 347 6. 6 mo. - n= 057 6. 4 mo. - n= 138 - 8. 4 mo. MEDIAN OVERALL SURVIVAL METASTATIC COLORECTAL CANCER NS

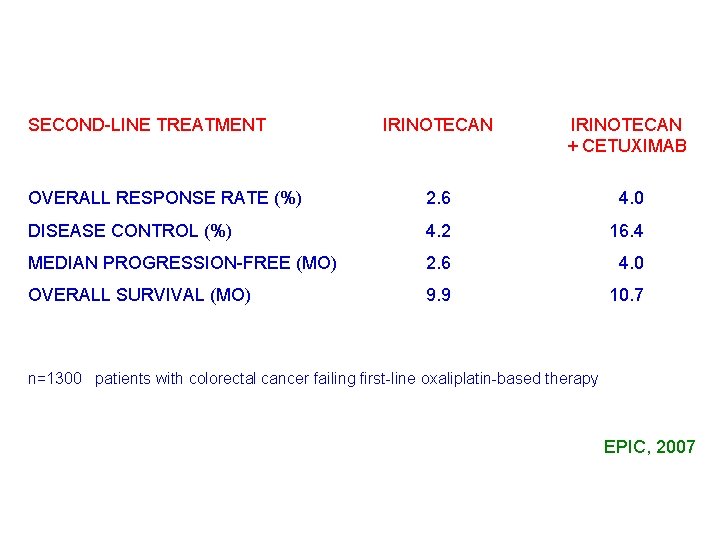
SECOND-LINE TREATMENT IRINOTECAN + CETUXIMAB OVERALL RESPONSE RATE (%) 2. 6 04. 0 DISEASE CONTROL (%) 4. 2 16. 4 MEDIAN PROGRESSION-FREE (MO) 2. 6 04. 0 OVERALL SURVIVAL (MO) 9. 9 10. 7 n=1300 patients with colorectal cancer failing first-line oxaliplatin-based therapy EPIC, 2007

NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Final Appraisal Determination Bevacizumab and cetuximab for metastatic colorectal Cancer Bevacizumab in combination with 5 -fluorouracil plus folinic acid, with or without irinotecan, is not recommended for the first-line treatment of metastatic colorectal cancer. Cetuximab in combination with irinotecan is not recommended for the secondline or subsequent treatment of metastatic colorectal cancer.

THERE IS A NEED FOR MORE INDEPENDENT CLINICAL RESEARCH • AT LEAST ONE PHASE 3 STUDY FOR DRUG APPROVAL SHOULD BE PERFORMED BY A NON-PROFIT INSTITUTION • HEAD TO HEAD COMPARISON OF SINGLE DRUGS OR STRATEGIES

THE ROLE OF AIFA • 54 PROJECTS APPROVED AND FINANCED IN 2006 (35 M €) • 51 PROJECTS APPROVED IN 2007

EXAMPLES OF APPROVED PROJECTS A prospective study on long-term outcome and potential usefulness of an intervention aimed at reducing adverse effects in patients with refractory epilepsy. Evaluation of prescribing pattern and safety profile of antidepressant and antipsychotic medications in italian general practice. Pharmacist’s outreach visits and new information formats: cluster and single-doctor randomised controlled trials for evaluating their feasibility and impact on knowledge, attitudes and prescribing practices of general practitioners in three italian regions.

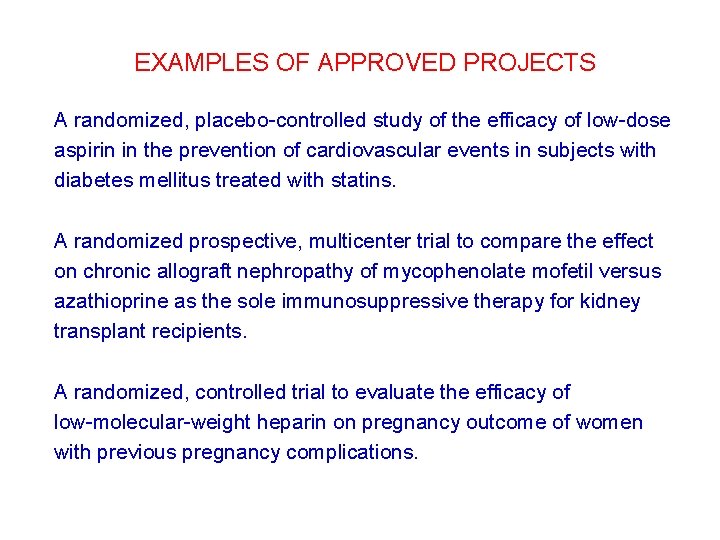
EXAMPLES OF APPROVED PROJECTS A randomized, placebo-controlled study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. A randomized prospective, multicenter trial to compare the effect on chronic allograft nephropathy of mycophenolate mofetil versus azathioprine as the sole immunosuppressive therapy for kidney transplant recipients. A randomized, controlled trial to evaluate the efficacy of low-molecular-weight heparin on pregnancy outcome of women with previous pregnancy complications.

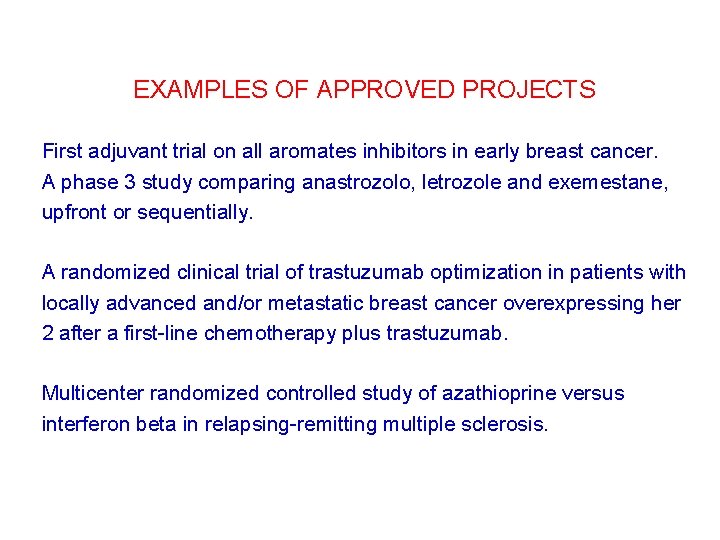
EXAMPLES OF APPROVED PROJECTS First adjuvant trial on all aromates inhibitors in early breast cancer. A phase 3 study comparing anastrozolo, letrozole and exemestane, upfront or sequentially. A randomized clinical trial of trastuzumab optimization in patients with locally advanced and/or metastatic breast cancer overexpressing her 2 after a first-line chemotherapy plus trastuzumab. Multicenter randomized controlled study of azathioprine versus interferon beta in relapsing-remitting multiple sclerosis.
 Liceo classico silvio pellico cuneo
Liceo classico silvio pellico cuneo Silvio santos figli
Silvio santos figli Silvio frascati
Silvio frascati Silvio teitelbaum
Silvio teitelbaum Silvio hiroshi nakao
Silvio hiroshi nakao Silvio cesare
Silvio cesare Silvio santos botox
Silvio santos botox Parafacias
Parafacias Silvio santos empreendedor
Silvio santos empreendedor Istituto comprensivo silvio pellico vedano olona
Istituto comprensivo silvio pellico vedano olona Liceo classico cuneo
Liceo classico cuneo Silvio tosatto
Silvio tosatto Lukas-kanade
Lukas-kanade Exhausted drug
Exhausted drug Intersect and minus in sql
Intersect and minus in sql This project is funded by the european union
This project is funded by the european union Co-funded by the erasmus+ programme of the european union
Co-funded by the erasmus+ programme of the european union This project is funded by the european union
This project is funded by the european union This project is funded by the european union
This project is funded by the european union European union military
European union military This project is funded by the european union
This project is funded by the european union Co-funded by the erasmus+ programme of the european union
Co-funded by the erasmus+ programme of the european union This project is co-funded by the european union
This project is co-funded by the european union European taekwondo union
European taekwondo union Borchert's epochs ap human geography definition
Borchert's epochs ap human geography definition European union history
European union history This project is funded by the european union
This project is funded by the european union European union 28 countries
European union 28 countries Eureka eurostars eligibility
Eureka eurostars eligibility Https://europa.eu/european-union/index_en
Https://europa.eu/european-union/index_en Functions of european union
Functions of european union Co-funded by the erasmus+ programme of the european union
Co-funded by the erasmus+ programme of the european union This project is funded by the european union
This project is funded by the european union European union 28 countries
European union 28 countries Navy zero tolerance drug policy
Navy zero tolerance drug policy Alm policy credit union
Alm policy credit union ưu thế lai là gì
ưu thế lai là gì Hệ hô hấp
Hệ hô hấp Tư thế ngồi viết
Tư thế ngồi viết Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Bổ thể
Bổ thể Tư thế ngồi viết
Tư thế ngồi viết V cc
V cc Thẻ vin
Thẻ vin Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu