DRUG DISCOVERY AND DEVELOPMENT Synthesis analog compounds and










- Slides: 42

DRUG DISCOVERY AND DEVELOPMENT “Synthesis analog compounds and Its Biological Activity” MUHAMMAD HANAFI Research Centre for. Chemistry (RC Chem) - LIPI

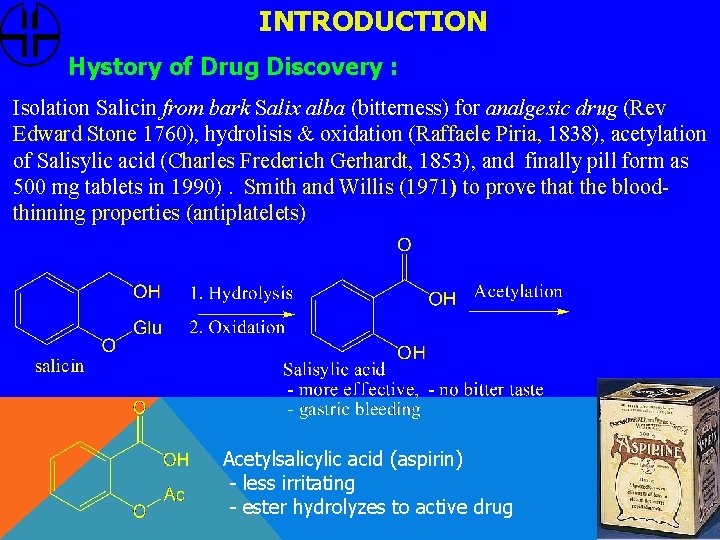
INTRODUCTION Hystory of Drug Discovery : Isolation Salicin from bark Salix alba (bitterness) for analgesic drug (Rev Edward Stone 1760), hydrolisis & oxidation (Raffaele Piria, 1838), acetylation of Salisylic acid (Charles Frederich Gerhardt, 1853), and finally pill form as 500 mg tablets in 1990). Smith and Willis (1971) to prove that the bloodthinning properties (antiplatelets) Acetylsalicylic acid (aspirin) - less irritating - ester hydrolyzes to active drug

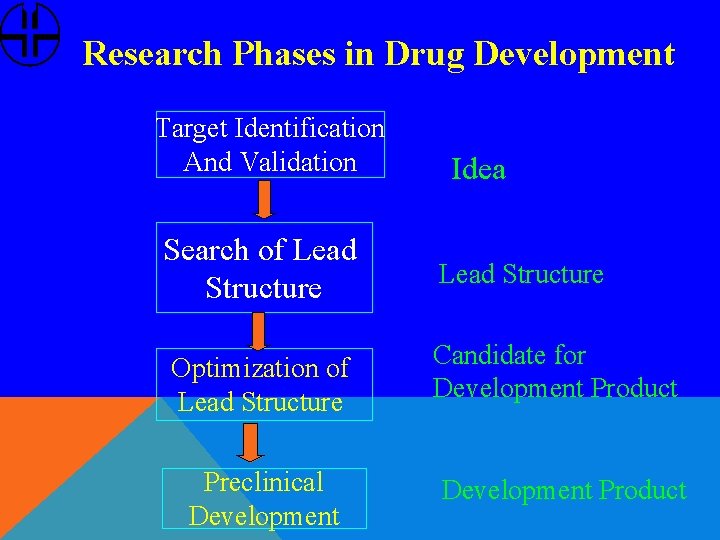
Research Phases in Drug Development Target Identification And Validation Idea Search of Lead Structure Optimization of Lead Structure Candidate for Development Product Preclinical Development Product

FOUR MAIN APPROACHES TO DISCOVERING NEW DRUGS 1. From Natural Products : Screening to find biologically active component 2. From the drugs in use : Modification to improve activity or to find different 3. From synthetic chemicals and animal models Screening of chemical library by disease animal models 4. From the modern approach to drug design Designing drugs based on physiological

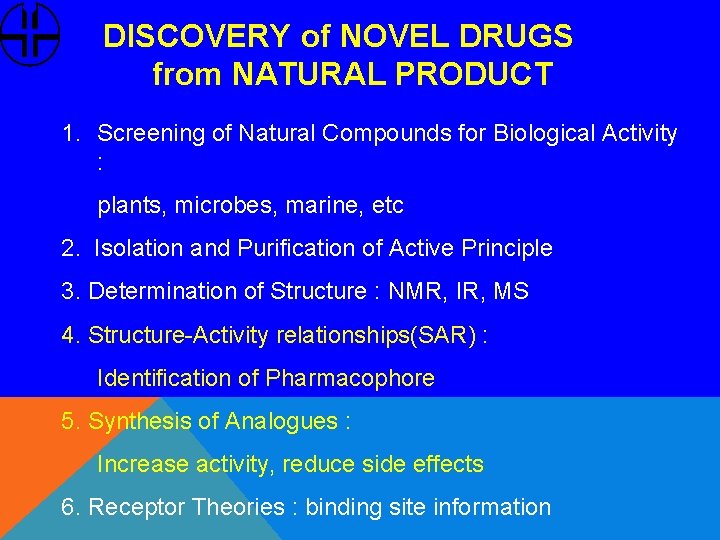
DISCOVERY of NOVEL DRUGS from NATURAL PRODUCT 1. Screening of Natural Compounds for Biological Activity : plants, microbes, marine, etc 2. Isolation and Purification of Active Principle 3. Determination of Structure : NMR, IR, MS 4. Structure-Activity relationships(SAR) : Identification of Pharmacophore 5. Synthesis of Analogues : Increase activity, reduce side effects 6. Receptor Theories : binding site information

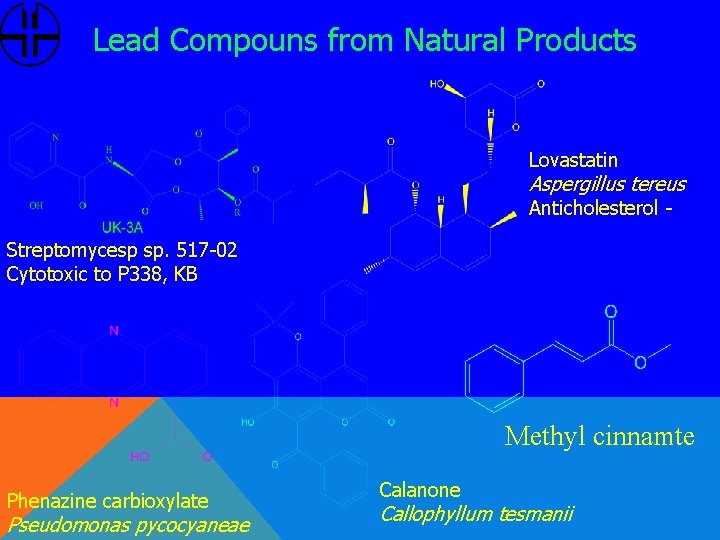
Lead Compouns from Natural Products Lovastatin Aspergillus tereus Anticholesterol Streptomycesp sp. 517 -02 Cytotoxic to P 338, KB Methyl cinnamte Phenazine carbioxylate Pseudomonas pycocyaneae Calanone Callophyllum tesmanii

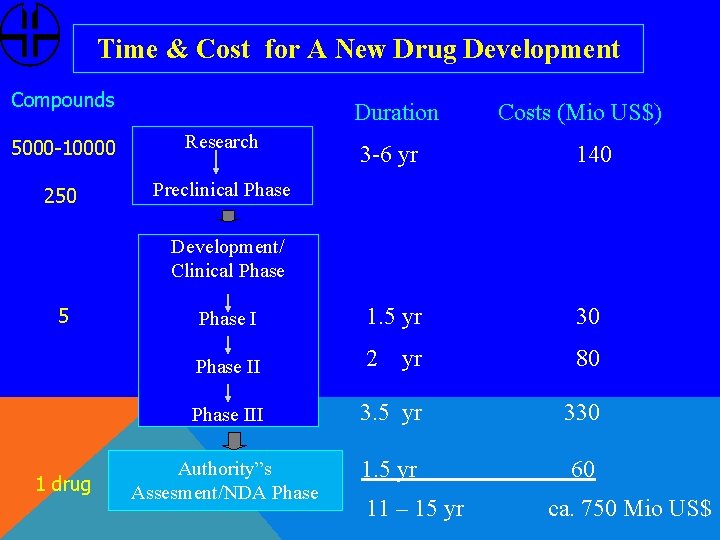
Time & Cost for A New Drug Development Compounds Duration 5000 -10000 Research 250 Preclinical Phase Costs (Mio US$) 3 -6 yr 140 Phase I 1. 5 yr 30 Phase II 2 yr 80 Phase III 3. 5 yr 330 Authority”s Assesment/NDA Phase 1. 5 yr 60 Development/ Clinical Phase 5 1 drug 11 – 15 yr ca. 750 Mio US$

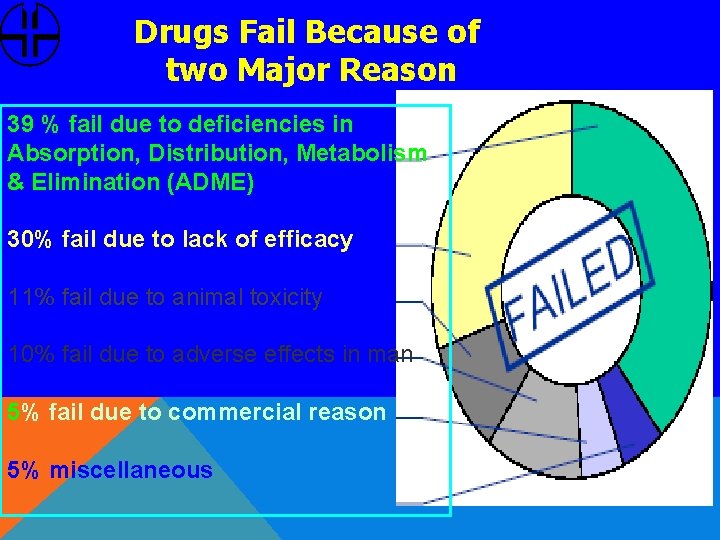
Drugs Fail Because of two Major Reason 39 % fail due to deficiencies in Absorption, Distribution, Metabolism & Elimination (ADME) 30% fail due to lack of efficacy 11% fail due to animal toxicity 10% fail due to adverse effects in man 5% fail due to commercial reason 5% miscellaneous

Lipinski’s “Rule of Five” Christopher Lipinski proposed four parameters that define the "drug- likeness" of potential drug candidates based on analysis of existing drug molecules. "The Rule of Five" got its name from the cut-off values for each of these parameters of which all have values of five or a multiple of five. The “rule” states that poor absorption or permeation is more likely when : –A compound has > 5 H-bond donors (sum of OHs and NHs); –There are > 10 H-bond acceptors (sum of Ns and Os); –The MW is > 500; –The. Log. P is > 5 (or MLog. P is over 4. 15). The “rule” is used by many as a useful guide in drug design.

The rule of five - formulation Poor absorption or permeation are more likely when: There are more than 5 H-bond donors. n The molecular weight is over 500. n The Log. P is over 5. n There are more than 10 H-bond acceptors. n

OPTIMAZATION ACTIVITY: SYNTHESIS OF DERIVATIVES/ ANALOGOUS

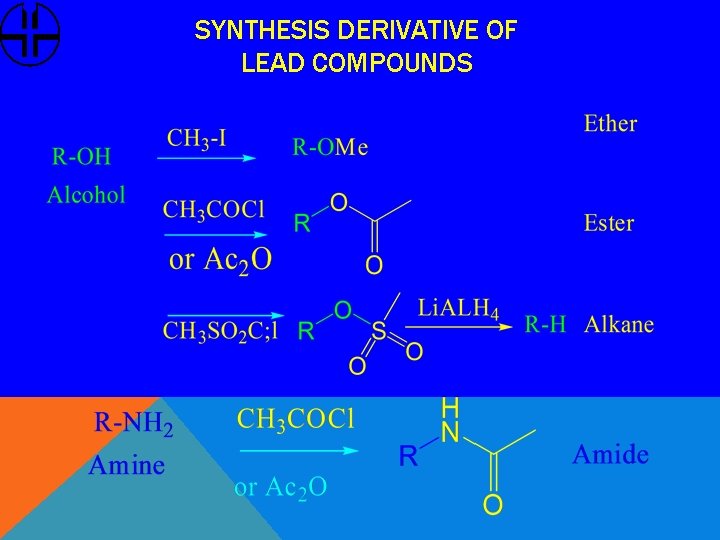
SYNTHESIS DERIVATIVE OF LEAD COMPOUNDS

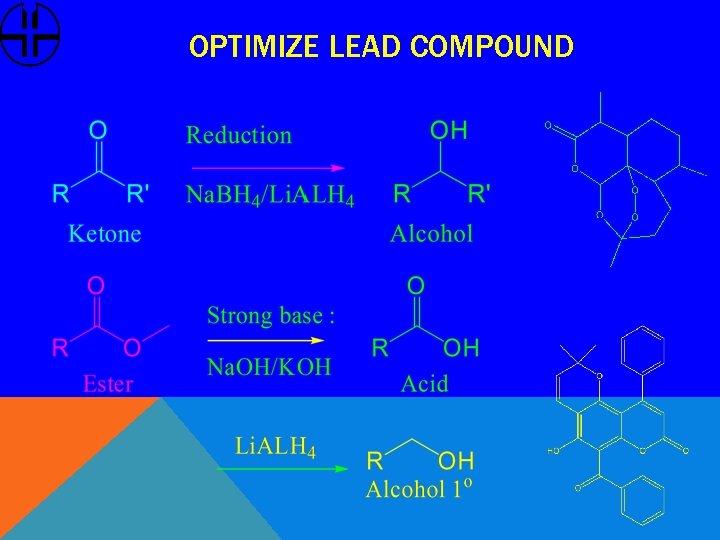
OPTIMIZE LEAD COMPOUND

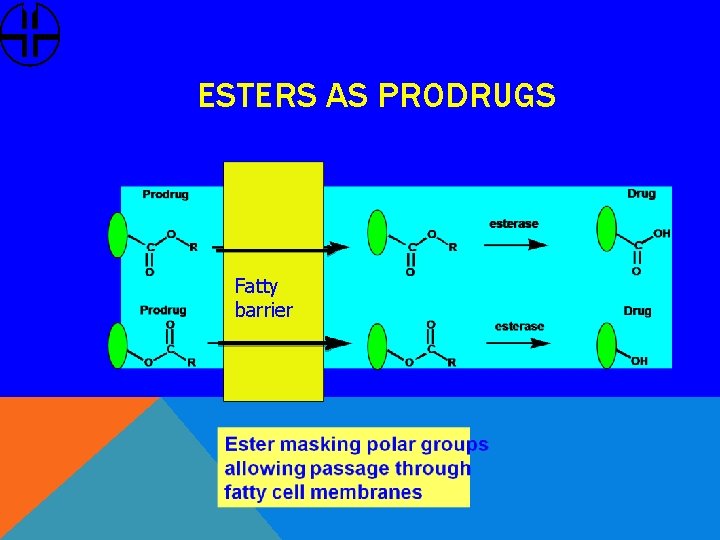
ESTERS AS PRODRUGS Fatty barrier

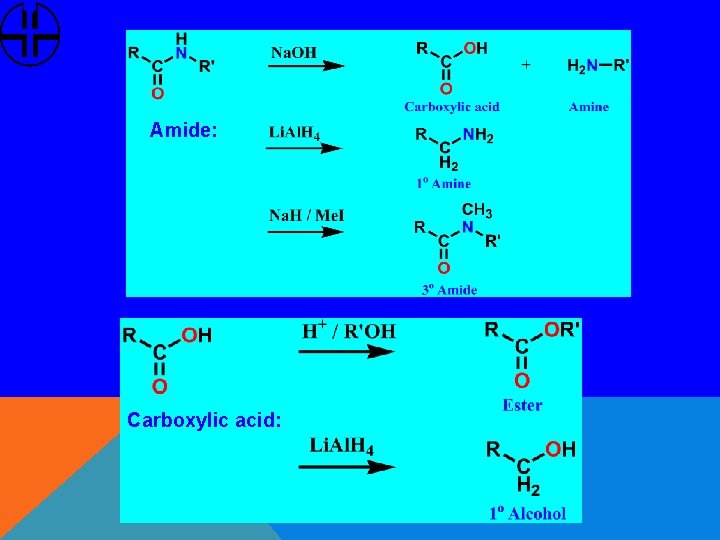
Amide: Carboxylic acid:

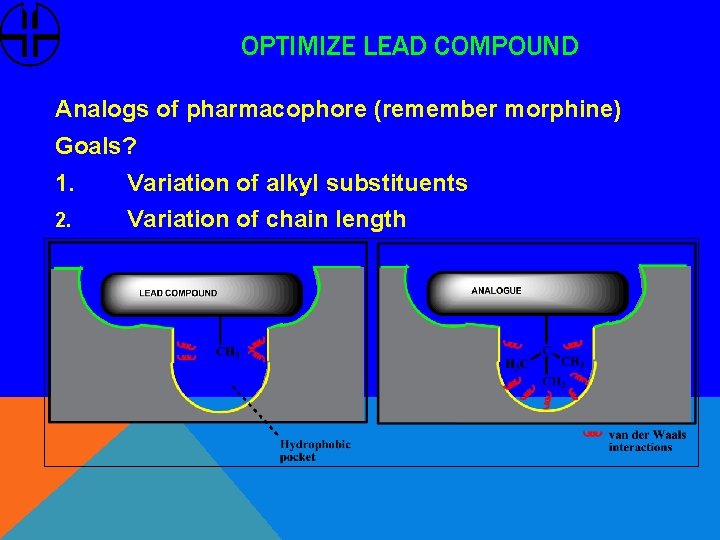
OPTIMIZE LEAD COMPOUND Analogs of pharmacophore (remember morphine) Goals? 1. Variation of alkyl substituents 2. Variation of chain length

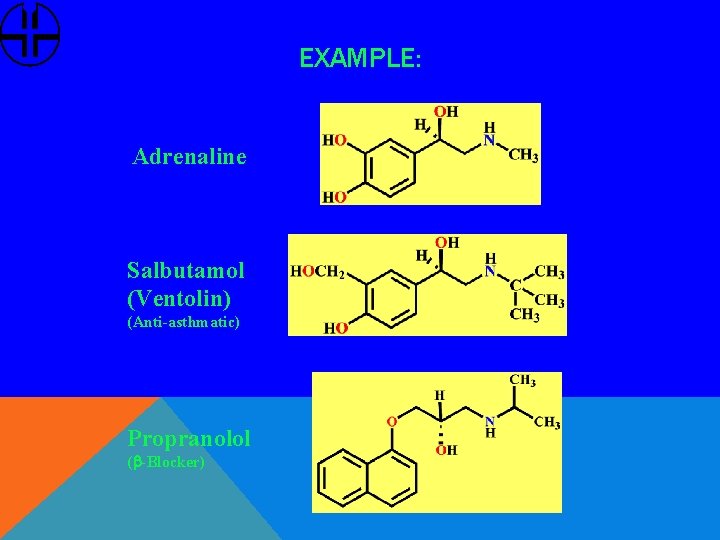
EXAMPLE: Adrenaline Salbutamol (Ventolin) (Anti-asthmatic) Propranolol (b-Blocker)

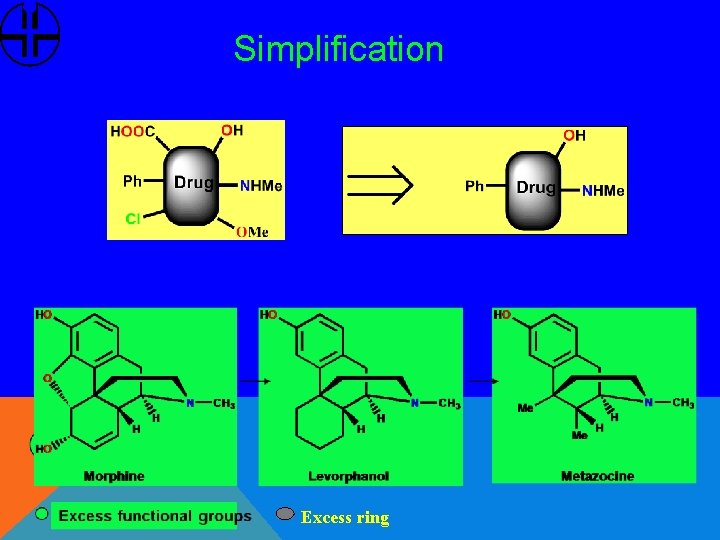
Simplification Excess ring

DRUGS /LEAD COMPOUNDS DEVELOPMENTS

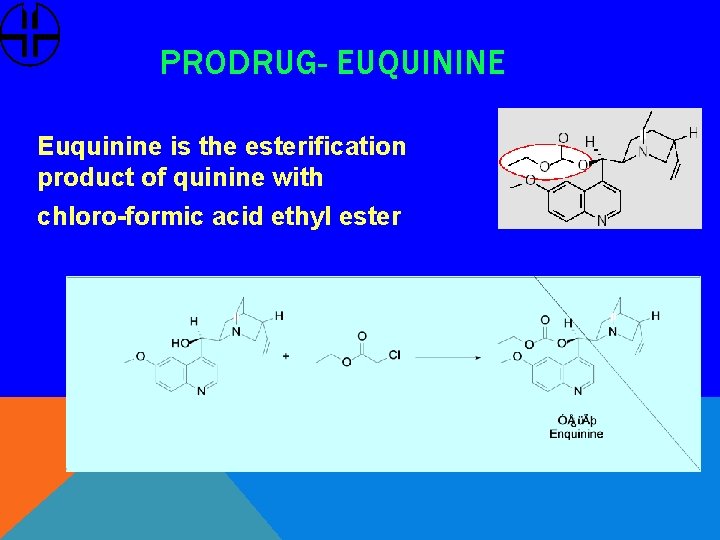
PRODRUG- EUQUININE Euquinine is the esterification product of quinine with chloro-formic acid ethyl ester

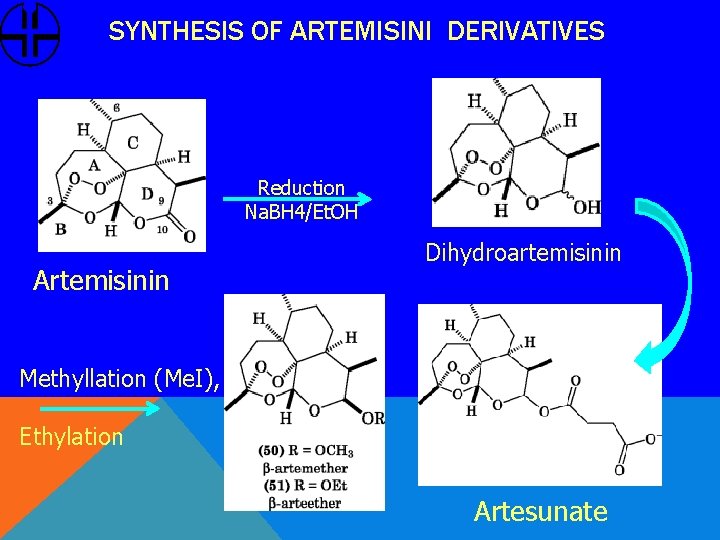
SYNTHESIS OF ARTEMISINI DERIVATIVES Reduction Na. BH 4/Et. OH Artemisinin Dihydroartemisinin Methyllation (Me. I), Ethylation Artesunate

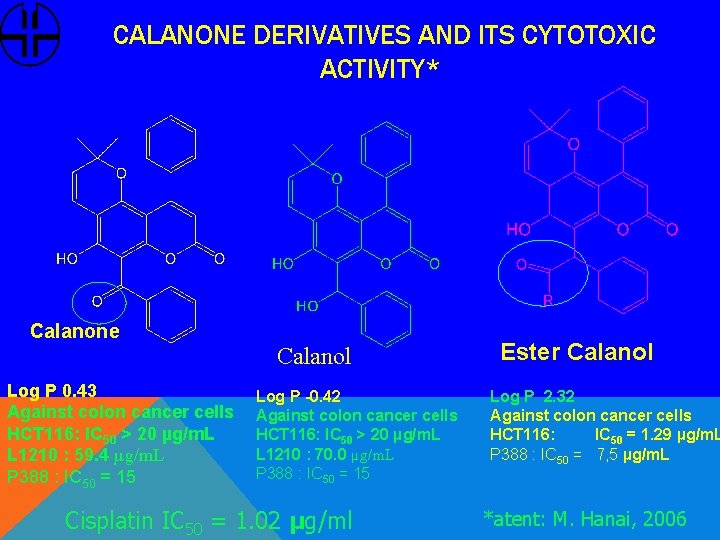
CALANONE DERIVATIVES AND ITS CYTOTOXIC ACTIVITY* Calanone Calanol Log P 0. 43 Against colon cancer cells HCT 116: IC 50 > 20 µg/m. L L 1210 : 59. 4 µg/m. L P 388 : IC 50 = 15 Log P -0. 42 Against colon cancer cells HCT 116: IC 50 > 20 µg/m. L L 1210 : 70. 0 µg/m. L P 388 : IC 50 = 15 Cisplatin IC 50 = 1. 02 µg/ml Ester Calanol Log P 2. 32 Against colon cancer cells HCT 116: IC 50 = 1. 29 µg/m. L P 388 : IC 50 = 7, 5 µg/m. L *atent: M. Hanai, 2006

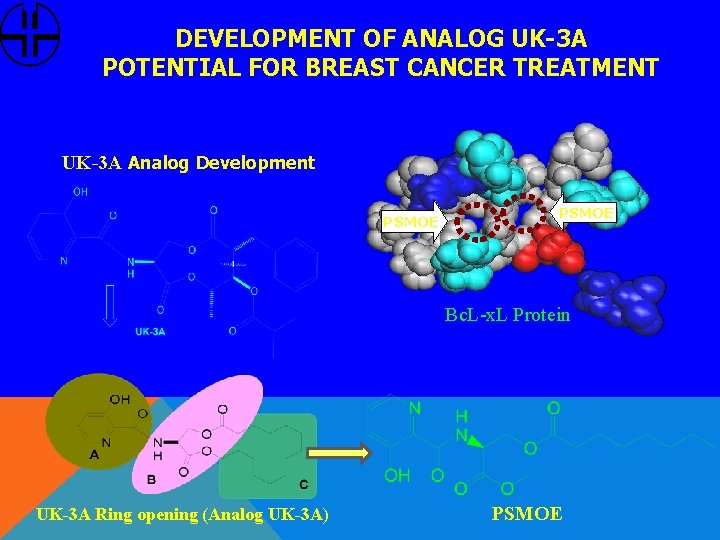
DEVELOPMENT OF ANALOG UK-3 A POTENTIAL FOR BREAST CANCER TREATMENT UK-3 A Analog Development PSMOE Bc. L-x. L Protein UK-3 A Ring opening (Analog UK-3 A) PSMOE

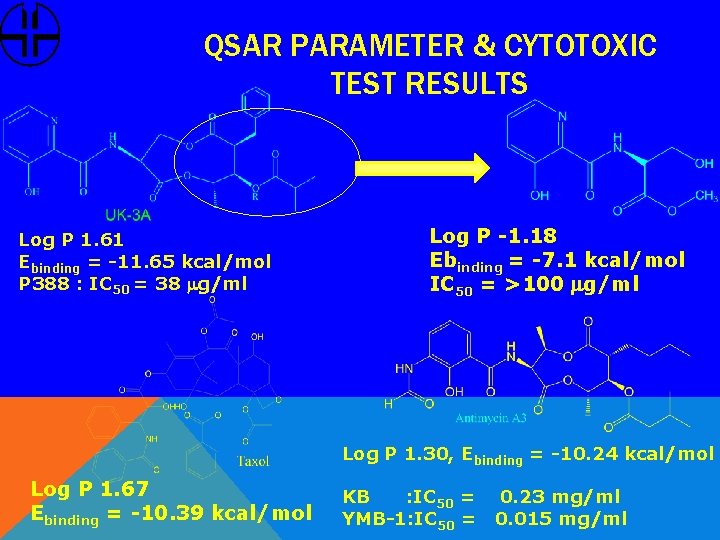
QSAR PARAMETER & CYTOTOXIC TEST RESULTS Log P 1. 61 Ebinding = -11. 65 kcal/mol P 388 : IC 50 = 38 mg/ml Log P -1. 18 Ebinding = -7. 1 kcal/mol IC 50 = >100 mg/ml Log P 1. 30, Ebinding = -10. 24 kcal/mol Log P 1. 67 Ebinding = -10. 39 kcal/mol KB : IC 50 = YMB-1: IC 50 = 0. 23 mg/ml 0. 015 mg/ml

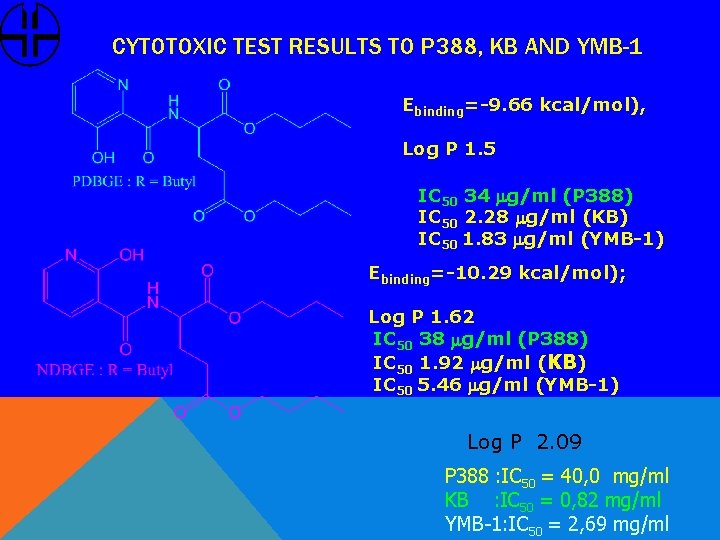
CYTOTOXIC TEST RESULTS TO P 388, KB AND YMB-1 Ebinding=-9. 66 kcal/mol), Log P 1. 5 IC 50 34 mg/ml (P 388) IC 50 2. 28 mg/ml (KB) IC 50 1. 83 mg/ml (YMB-1) Ebinding=-10. 29 kcal/mol); Log P 1. 62 IC 50 38 mg/ml (P 388) IC 50 1. 92 mg/ml (KB) IC 50 5. 46 mg/ml (YMB-1) Log P 2. 09 P 388 : IC 50 = 40, 0 mg/ml KB : IC 50 = 0, 82 mg/ml YMB-1: IC 50 = 2, 69 mg/ml

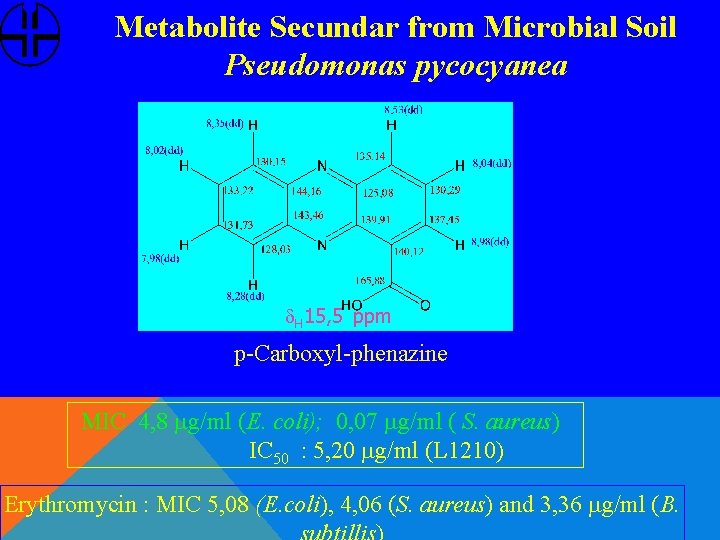
Metabolite Secundar from Microbial Soil Pseudomonas pycocyanea d. H 15, 5 ppm p-Carboxyl-phenazine MIC 4, 8 mg/ml (E. coli); 0, 07 mg/ml ( S. aureus) IC 50 : 5, 20 mg/ml (L 1210) Erythromycin : MIC 5, 08 (E. coli), 4, 06 (S. aureus) and 3, 36 mg/ml (B.

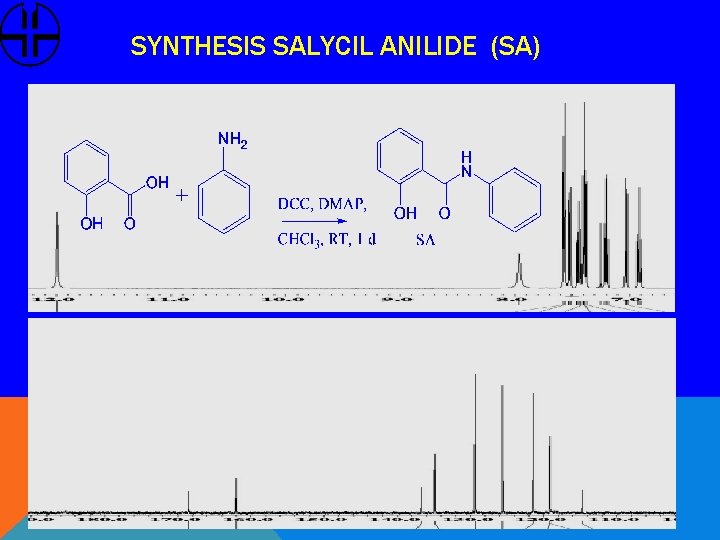
SYNTHESIS SALYCIL ANILIDE (SA)

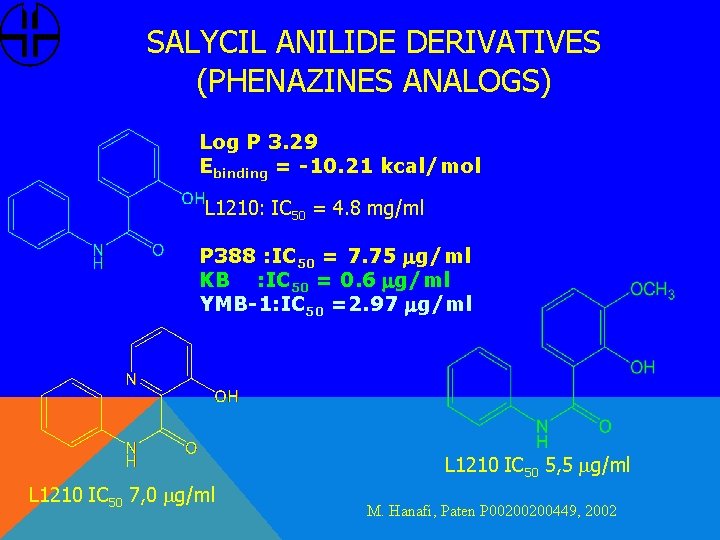
SALYCIL ANILIDE DERIVATIVES (PHENAZINES ANALOGS) Log P 3. 29 Ebinding = -10. 21 kcal/mol L 1210: IC 50 = 4. 8 mg/ml P 388 : IC 50 = 7. 75 mg/ml KB : IC 50 = 0. 6 mg/ml YMB-1: IC 50 =2. 97 mg/ml L 1210 IC 50 5, 5 mg/ml L 1210 IC 50 7, 0 mg/ml M. Hanafi, Paten P 00200200449, 2002

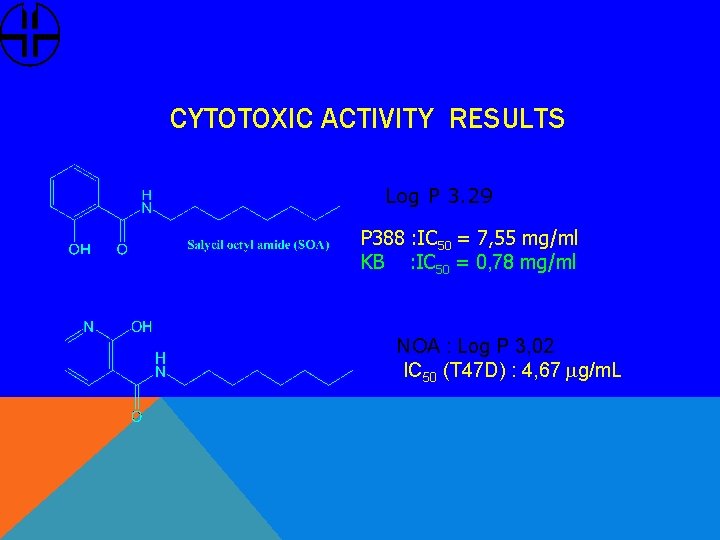
CYTOTOXIC ACTIVITY RESULTS Log P 3. 29 P 388 : IC 50 = 7, 55 mg/ml KB : IC 50 = 0, 78 mg/ml NOA : Log P 3, 02 IC 50 (T 47 D) : 4, 67 mg/m. L

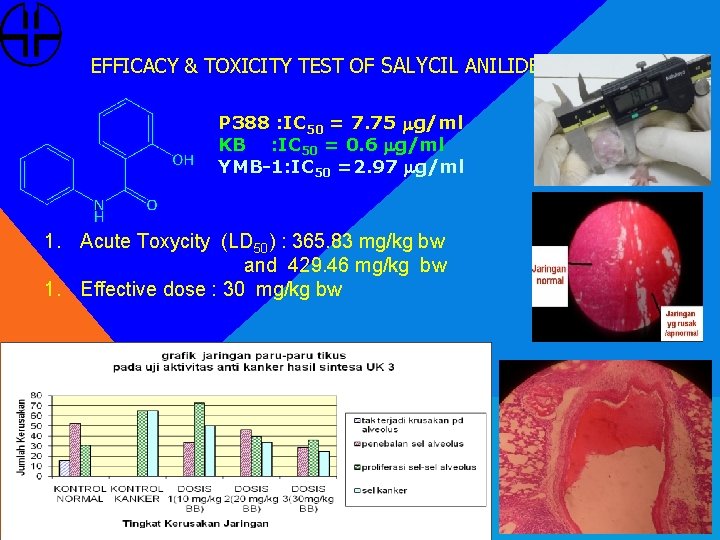
EFFICACY & TOXICITY TEST OF SALYCIL ANILIDE (SA) P 388 : IC 50 = 7. 75 mg/ml KB : IC 50 = 0. 6 mg/ml YMB-1: IC 50 =2. 97 mg/ml 1. Acute Toxycity (LD 50) : 365. 83 mg/kg bw and 429. 46 mg/kg bw 1. Effective dose : 30 mg/kg bw a

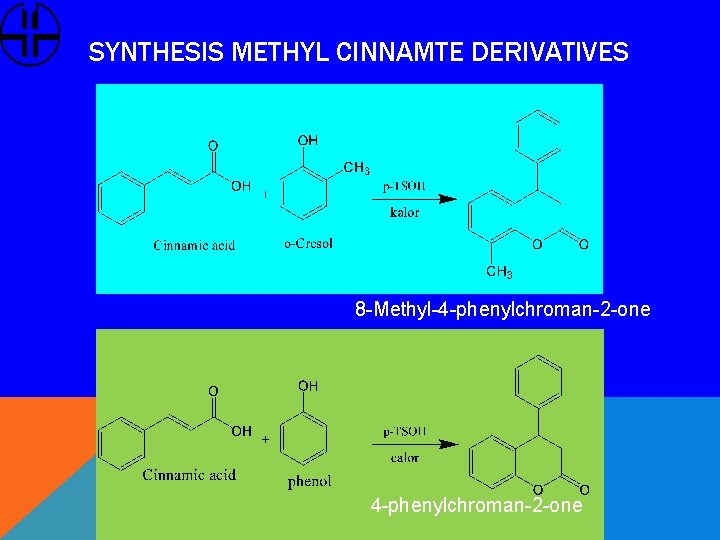
SYNTHESIS METHYL CINNAMTE DERIVATIVES 8 -Methyl-4 -phenylchroman-2 -one

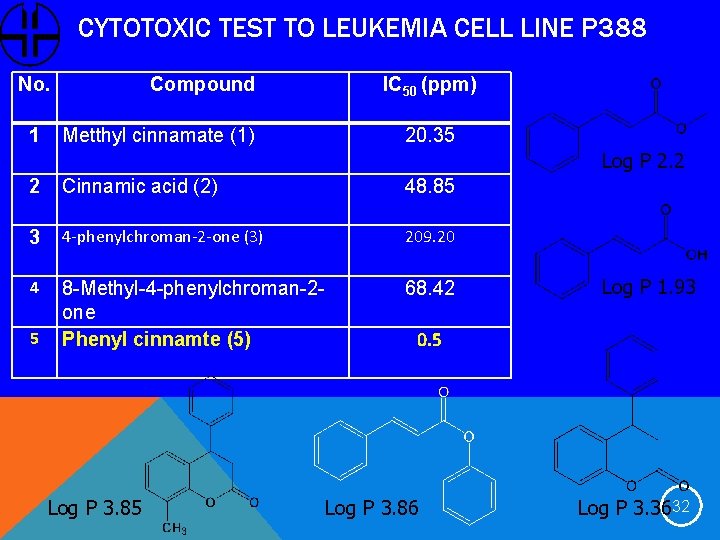
CYTOTOXIC TEST TO LEUKEMIA CELL LINE P 388 No. 1 Compound Metthyl cinnamate (1) IC 50 (ppm) 20. 35 Log P 2. 2 2 Cinnamic acid (2) 48. 85 3 4 -phenylchroman-2 -one (3) 209. 20 4 8 -Methyl-4 -phenylchroman-2 one Phenyl cinnamte (5) 68. 42 5 Log P 3. 85 Log P 1. 93 0. 5 Log P 3. 86 Log P 3. 3632

FIND AND OPTIMIZED A LEAD COMPOUND: LOVASTATIN » Minimise energy of structure : Chem 3 D, Gaussian, Mopac, » Structure Activy Correlationship : Hyper. Chem. Pro » Direct Ligand Design (HMG-Co. A rductase): Arguslab 4. 0 » Synthesis » Bioaactivity Test

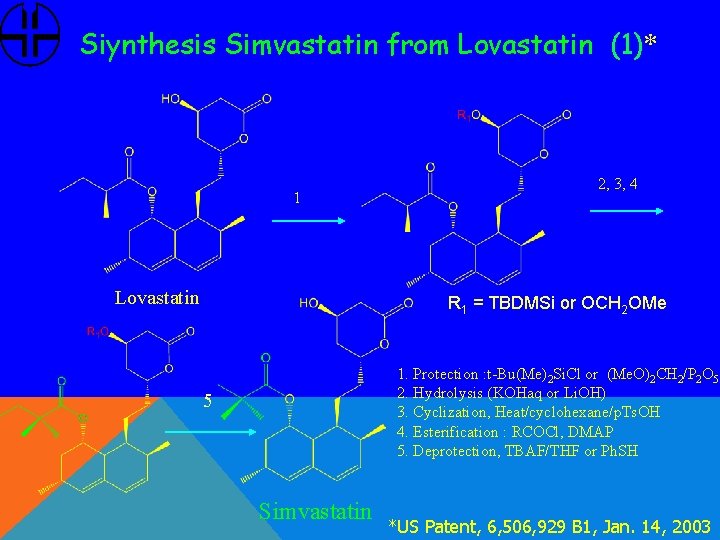
Siynthesis Simvastatin from Lovastatin (1)* 1 Lovastatin 2, 3, 4 R 1 = TBDMSi or OCH 2 OMe 1. Protection : t-Bu(Me)2 Si. Cl or (Me. O)2 CH 2/P 2 O 5 2. Hydrolysis (KOHaq or Li. OH) 3. Cyclization, Heat/cyclohexane/p. Ts. OH 4. Esterification : RCOCl, DMAP 5. Deprotection, TBAF/THF or Ph. SH 5 Simvastatin *US Patent, 6, 506, 929 B 1, Jan. 14, 2003

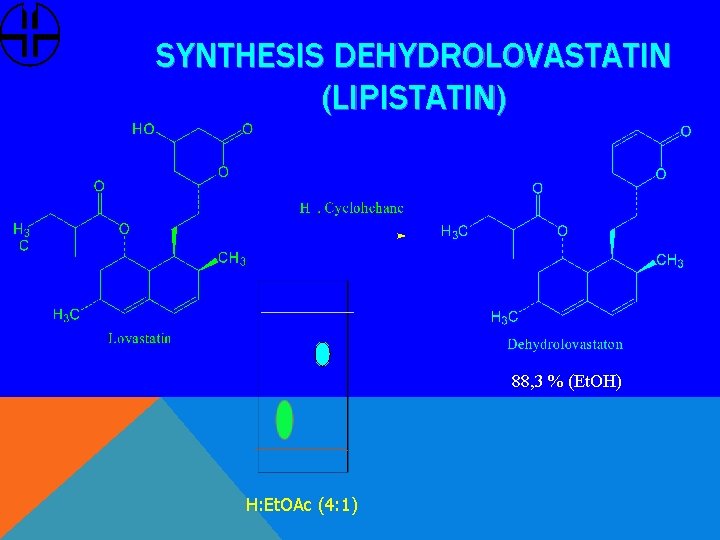
SYNTHESIS DEHYDROLOVASTATIN (LIPISTATIN) 88, 3 % (Et. OH) H: Et. OAc (4: 1)

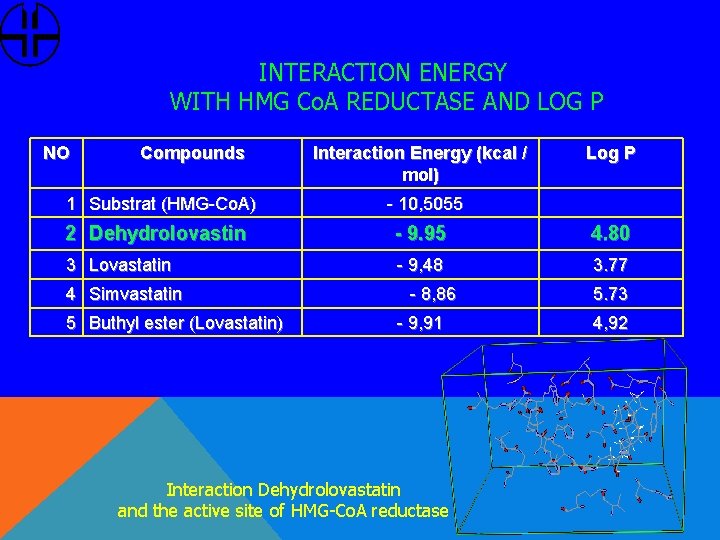
INTERACTION ENERGY WITH HMG Co. A REDUCTASE AND LOG P NO Compounds 1 Substrat (HMG-Co. A) Interaction Energy (kcal / mol) Log P - 10, 5055 2 Dehydrolovastin - 9. 95 4. 80 3 Lovastatin - 9, 48 3. 77 4 Simvastatin 5 Buthyl ester (Lovastatin) - 8, 86 - 9, 91 Interaction Dehydrolovastatin and the active site of HMG-Co. A reductase 5. 73 4, 92

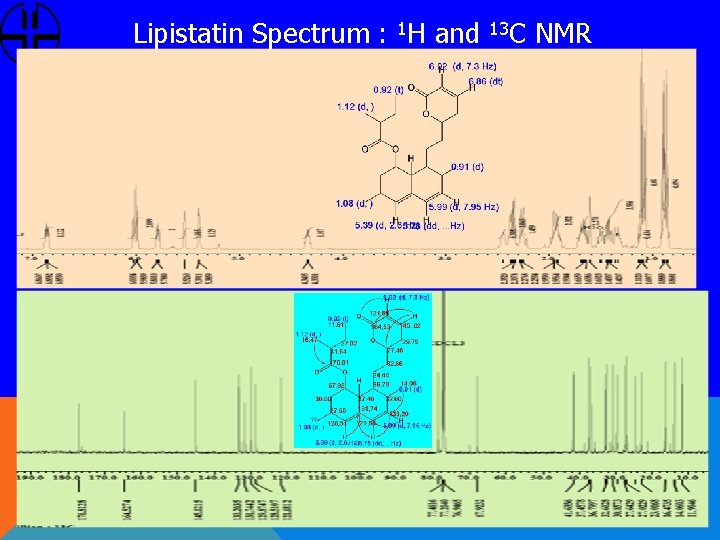
Lipistatin Spectrum : 1 H and 13 C NMR

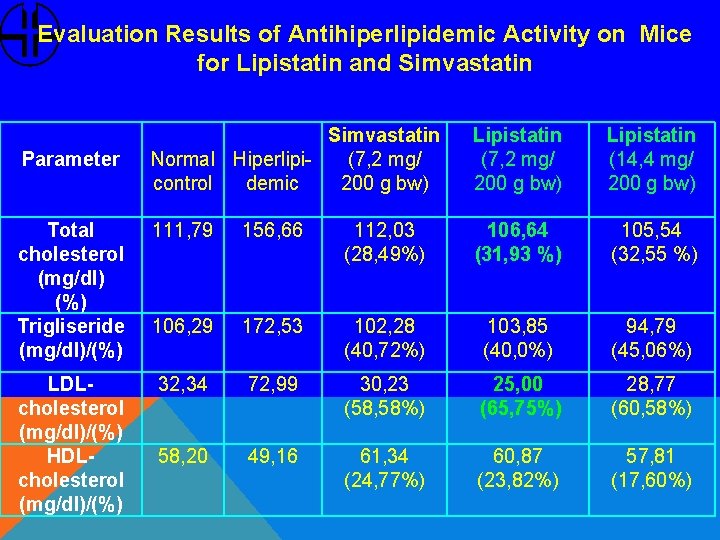
Evaluation Results of Antihiperlipidemic Activity on Mice for Lipistatin and Simvastatin Normal Hiperlipi(7, 2 mg/ control demic 200 g bw) Lipistatin (7, 2 mg/ 200 g bw) Lipistatin (14, 4 mg/ 200 g bw) Total cholesterol (mg/dl) (%) Trigliseride (mg/dl)/(%) 111, 79 156, 66 112, 03 (28, 49%) 106, 64 (31, 93 %) 105, 54 (32, 55 %) 106, 29 172, 53 102, 28 (40, 72%) 103, 85 (40, 0%) 94, 79 (45, 06%) LDLcholesterol (mg/dl)/(%) HDLcholesterol (mg/dl)/(%) 32, 34 72, 99 30, 23 (58, 58%) 25, 00 (65, 75%) 28, 77 (60, 58%) 58, 20 49, 16 61, 34 (24, 77%) 60, 87 (23, 82%) 57, 81 (17, 60%) Parameter

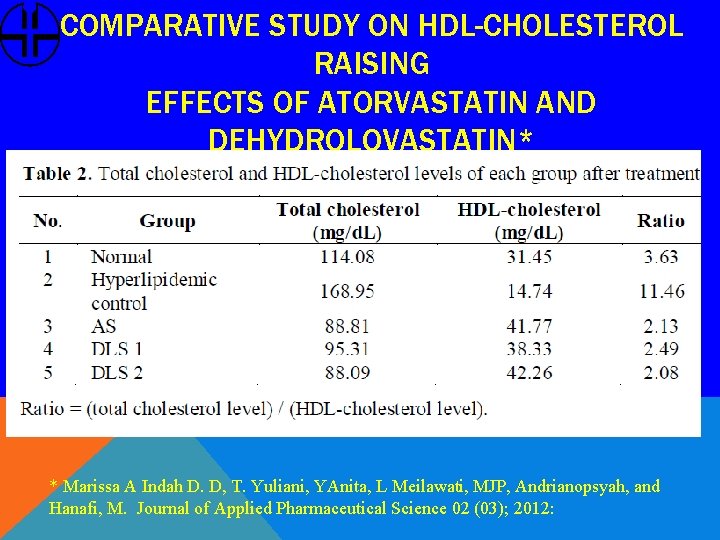
COMPARATIVE STUDY ON HDL-CHOLESTEROL RAISING EFFECTS OF ATORVASTATIN AND DEHYDROLOVASTATIN* * Marissa A Indah D. D, T. Yuliani, YAnita, L Meilawati, MJP, Andrianopsyah, and Hanafi, M. Journal of Applied Pharmaceutical Science 02 (03); 2012:

CONCLUSION 1. To get a new drug is very complex, take time, and costly 2. Starting material (lead comp) could be isolated from the major comps. 3. The Lipinski’s “Rule of Five is used by many as a useful guide in drug design. 4. To optimized acrtivity of lead compouunds can be make derivatives, by simple methods: methylation. reduction, esterification, hydrolisis, and simplification 5. Lipofilicity FG is important for biological activity 6. Analog UK-3 A were potential candidate anticancer 7. Dehydrolovastatin is potential a new candidate drug for anticholesterol

ACKNOLEDMENTS ü Indonesian Institute of Science (LIPI) & Ministry of Sci & Tech (KNRT) and JSPS for fund ü RC Chem LIPI for support facilities ü Osaka City Univeristy Japan for cytotoxic test

TERIMAKASIH