Drug discovery and development Drug discovery and development




















- Slides: 28

Drug discovery and development

Drug discovery and development Objectives of next 5 lectures: you will: �be aware of why/how new drugs are discovered �know the processes involved in drug discovery and development �see where pharmacologists/bioscientists may contribute �know about the difficulties and dangers inherent in the drug development process.

What costs what in Leeds? (GPs; 98/99) �Omeprazole (anti-gastric acid) £ 3. 5 m �Simvastatin (cholesterol lowering) £ 2. 4 m �Beclomethasone (asthma) £ 1. 8 m �Fluoxetine (antidepressant) £ 1. 5 m �Lansoprazole (anti-gastric acid) £ 1. 4 m �Ranitidine (anti-gastric acid) £ 1. 3 m �Paroxetine (antidepressant) £ 1. 2 m �TOP 7 TOTAL >£ 13 m �Total GP drugs for Leeds >£ 67 m

Why are new drugs needed? �unmet medical need; new diseases (BSE; AIDS, Alzheimer’s; obesity); low efficacy (dementia, cancer); side effects (antidepressants, antipsychotics) �downstream health costs; (Alzheimer’s; spinal injury) �cost of therapy; (Viagra, Interleukins) �costs to individual/country; (depression) �sustain industrial activity; pharmaceutical industry employs thousands and makes a massive contribution to overseas earnings); patent expiry

The changed context of drug discovery and development The 1800 s: natural sources; limited possibilities; prepared by individuals; small scale; not purified, standardised or tested; limited administration; no controls; no idea of mechanisms. The 1990 s: synthetic source; unlimited possibilities; prepared by companies; massive scale; highly purified, standardised and tested; world-wide administration; tight legislative control; mechanisms partly understood.

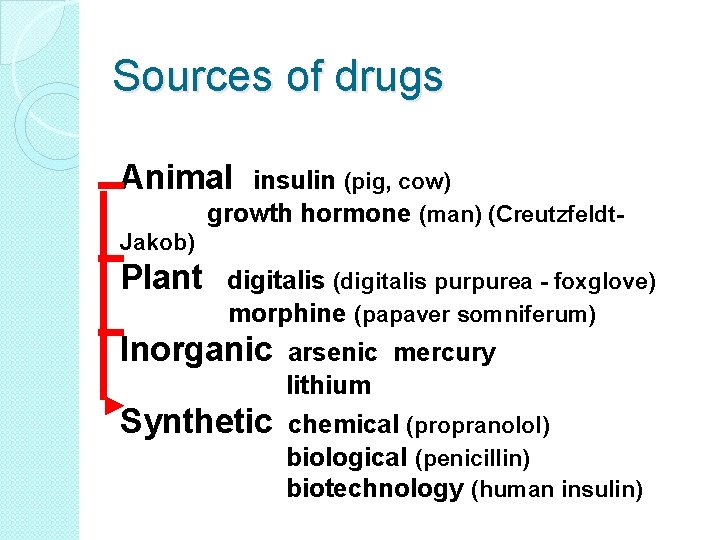
Sources of drugs Animal insulin (pig, cow) growth hormone (man) (Creutzfeldt- Jakob) Plant digitalis (digitalis purpurea - foxglove) morphine (papaver somniferum) Inorganic arsenic mercury lithium Synthetic chemical (propranolol) biological (penicillin) biotechnology (human insulin)

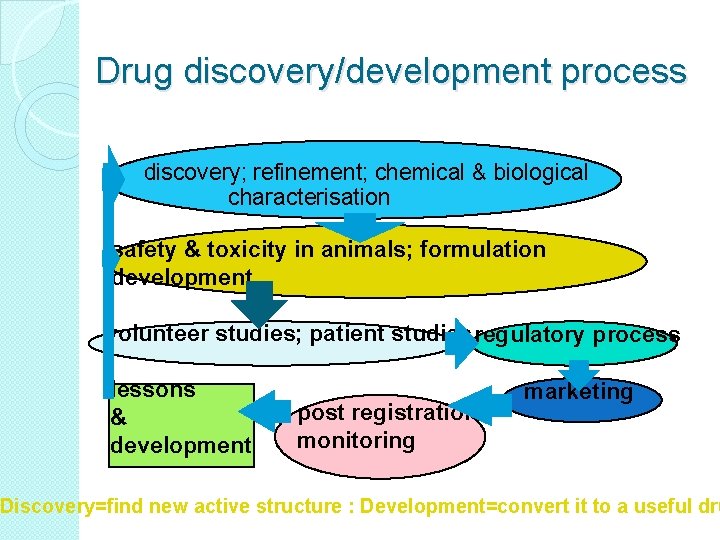
Drug discovery/development process discovery; refinement; chemical & biological characterisation safety & toxicity in animals; formulation development volunteer studies; patient studies regulatory process lessons & development post registration monitoring marketing Discovery=find new active structure : Development=convert it to a useful dru

Approaches to drug discovery �Historical; cinchona (quinine) & willow barks (aspirin); chinese medicine currently. �Study disease process; breast cancer (tamoxifen); �Study biochem/physiological pathway; Parkinson’s disease (L-dopa) renin/angiotensin �Develop SAR to natural compound; beta- adrenoceptors (propranolol), H 2 -receptors (cimetidine) �Design to fit known structurally identified biological site; angiotensin-converting enzyme inhibitors �By chance (serendipidy); random screening (HTS); penicillin; dimenhydramate; pethidine �Genomics; identification of receptors; gene therapy; recombinant materials; DRIVER IS UNMET MEDICAL NEED IN A VIABLE MARKET

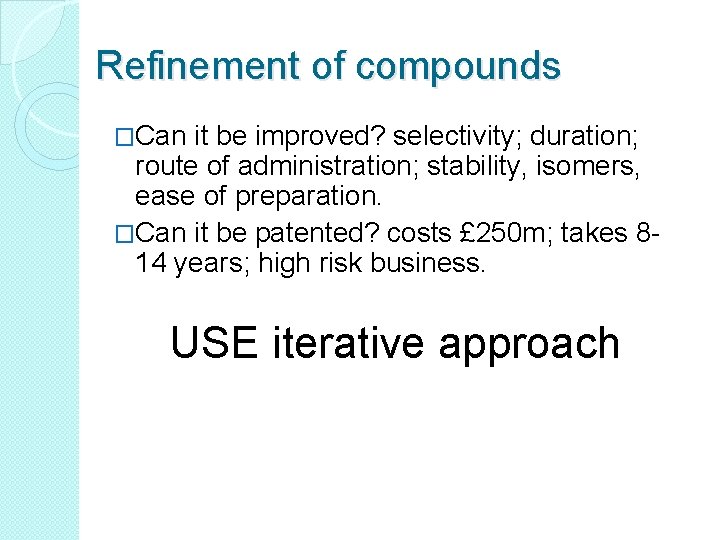
Refinement of compounds �Can it be improved? selectivity; duration; route of administration; stability, isomers, ease of preparation. �Can it be patented? costs £ 250 m; takes 814 years; high risk business. USE iterative approach

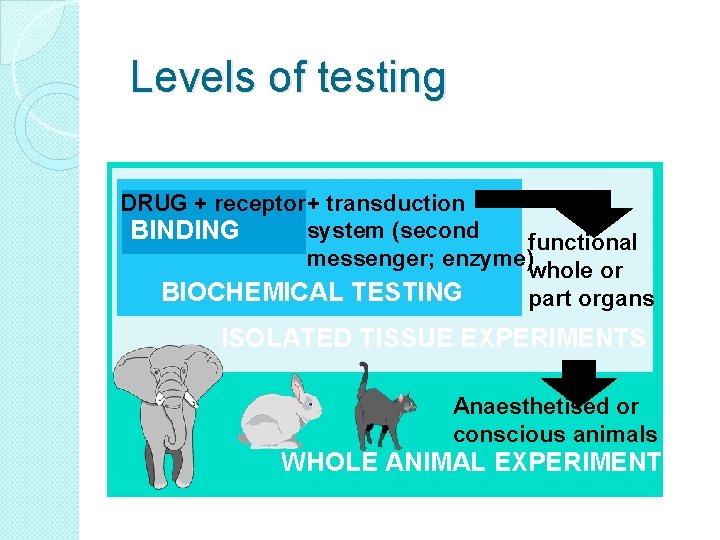
Levels of testing DRUG + receptor+ transduction system (second BINDING functional messenger; enzyme) whole or BIOCHEMICAL TESTING part organs ISOLATED TISSUE EXPERIMENTS Anaesthetised or conscious animals WHOLE ANIMAL EXPERIMENTS

Animal models of efficacy � Existing normal behaviours/effects (anaesthesia; contraception; paralysis) � Create behaviours (fat rats; hypertensive rats; anxious rats; epileptic rats) � Find unrelated behaviour affected by existing drugs (Straub tail for narcotic analgesics; learned helplessness for antidepressants) How predictive is the model? exact replica = 100% predictor mechanism same = good predictor mechanisms different = poor predictor

Animal models �predictive for efficacy AND toxicity? �expensive; time consuming; variable; uncertain; troublesome; ethical questions; skilled workers �legislative control Animal (Scientific Procedures) Act (1986) �PERSONAL LICENCE - competent, trained, procedures specified �PROJECT LICENCE - allows a personal licence holder to carry out specified procedures for a specified project that cannot be done without animals and where severity justifies likely gain. �GET INTO MAN EARLY

Reducing animal usage �About 2. 6 m animals/y used in procedures in UK (11. 6 m in Europe) �Likely to increase; more research, more targets, genetic capability � 3 Rs -- 3 Rs �REPLACEMENT: use non-animal tests if possible (cheaper, less trouble, less variable but not possible for everything at this time) �REDUCTION: get the statistics right, don’t replicate work unnecessarily, don’t overbreed �REFINEMENT: reduce suffering and severity of procedure, pay attention to housing, stress, husbandry and rich

Chemical and biological characterisation �CHEMICAL; structure, synthesis, purity, isomers, p. Ka, stability, solubility, salts, assay �BIOLOGICAL; acute pharmacological profile - LD 50, ED 50, binding data for many receptors, dose-effect relationships, open field tests, particular tests for different activities (e. g. CVS, CNS, GI tract) Both positive and negative information is useful.

Safety & toxicity in animals �Acute toxicity profile �Chronic toxicity profile -- 14 day toxicity test in one rodent and one non-rodent species before use in man. -- 3 month study read out at 28 days -- longer studies (12 & 24 month) Three dose levels (below, about, well above human dose). It is insufficient to to use doses which are not toxic; the doses producing toxic effects and the nature of these effects MUST be established.

Formulation studies �DRUG + Additive: filler, lubricant, coating, stabiliser, colour, binder, disintegrator Dosage form: capsule, tablet, injection, other? Manipulate duration/profile: e. g. sustained release Bioequivalence Bioavailability Ease of use

Clinical testing �{Phase 0 (non-clinical)} �Phase 1 (volunteers) �Phase 2 (patients) �Phase 3 (large scale multi-centre) �Phase 4 (post registration monitoring) phases can also be defined by the information you are trying to get out of the testing

Volunteer studies (phase I trials) �pharmacologists & employees (15 -30 in number) �ethical approval �healthy �informed consent �full rescussitation + medical backup �monitor �single and repeat doses �increase dose levels

Volunteer studies (phase I trials) OBJECTIVES �metabolic and excretory pathways (impinges on toxicity testing in animals) �variability between individuals; effect of route; bioavailability �tolerated dose range �indication of therapeutic effects �indication of side effects

Patient studies (phase 2 trials) � 150 -350 ill people; informed consent �needs licence �maximum monitoring; full rescussitation �often patients where other treatment failed �OBJECTIVES: indication for use; type of patient; severity of disease; dose range, schedule and increment; pharmacokinetic studies in ill people; nature of side effects and severity; effects in special groups.

Patient studies (phase 3 trials) � 1500 -3500 ill patients �multicentre? �more certain data for the objectives of phase 2 studies �interactions between drugs start to become measurable in the larger population �sub-groups start to be established �special features and problems show up

Clinical trials Drug action depends on: �pharmacodynamics �pharmacokinetics and dose regimen �drug interactions �receptor sensitivity of patient �mood/personality of patient & doctor �patients expectations and past experience �social environment of patient �clinical state of patient Clinical trial controls these variables and examines action of drug in defined set of circumstances

The Regulatory process �differs from country to country �demands safety and quality of product �encourages efficacy and need for product �grants clinical trials certificate if volunteer and animal data OK �approves protocols and examines data � 50 -400 volumes (30, 000 -150, 000 pages) �original data available �two way process; authority and company trying to produce a safe effective product �release for a specific purpose and use

Marketing �getting the product right (packaging; formulation) �right therapeutic slot �information on new drug �information for honest comparison �reporting problems �reporting new indications �therapeutic trends

Post-registration monitoring �YELLOW CARD SYSTEM: voluntary reporting of adverse effects by GP to Committee on Safety of Medicines; easy; effective? �INTENSIVE MONITORING OF DEFINED GROUP: first 10, 000; administrative nightmare as patients move/die; costly; timeconsuming. �RESTRICTED RELEASE: only available to small group of GPs; monitor their patients; elitist �MONITOR INCIDENCE OF DISEASE PROBLEM: difficult to identify cause of

Lessons and development �refine parts of treatment giving problems (dose interval? side effects? effective? niche market? ) �extend usage eg. PROPRANOLOL (beta adrenoceptor blocker) antidysrhythmic >>> antianginal >>> antihypertensive >>> relieve hyperthyroid symptoms >>> antihypertensive with diuretic >>> prolonged release formulation precipitate asthma attack > beta 1 selective ATENOLOL

The future? � 3 rd world diseases? �orphan drugs with few users? �improve safety and efficacy records �reduce animal utilisation (cell lines; early human volunteers, ) �new diseases (AIDS; Alzheimer’s; CJ disease; human BSE variant; obesity; cancer) �new biology - (clone human receptors; disease model by gene changes) �patent times and increasing cost

Me-too drugs Similar to drugs already on market �parallel co-incident development �not identical - differences emerge with time �allergy to one only �unsuspected side effect causes discontinuation �particular indication in sub-group of patients �sometimes too many