Drug Development Process Stages involved in Regulating Drugs







- Slides: 8

Drug Development Process Stages involved in Regulating Drugs Clinical Trials Eli. S Lec. No. 6 1

Clinical Trials - IND Phase 1 Initial Introduction into Human (20 -100 Healthy volunteers) Dose Ranging determined the maximum-tolerated dose with potential toxicities well-defined Closely Monitoring Eli. S Lec. No. 6 Safety 2

IND Phase 2 Controlled Clinical Trials Effectiveness (Preliminary) Defines the dose regimen of the drug Closely Monitoring Relatively Small Numbers (100 -300 Patient volunteers) Eli. S Lec. No. 6 3

IND Phase 2 (Cont’) n End-of-Phase 2 Meeting • Package received 30 days before scheduled date • Outstanding Nonclinical/CMC issues • Proposed Phase 3 adequate and well-controlled study design and analysis plan • Obtain agreement from FDA on Phase 3 adequate ( )מספיק and well-controlled study design and analysis plan n Adequate and well-controlled study • Has agreed-upon adequate and well-controlled design • Provides the data the FDA will base its go/no-go decision on • Must meet high scientific standards: controlled, blinded, randomized, adequate size Eli. S Lec. No. 6 4

IND Phase 3 Verifying efficacy, establishing safety, and establishing the optimum dosage Larger Studies (1000 -3000 Patient volunteers) Controlled Pivotal : ( )הכרעה To provide the data sufficient to convince the FDA of the favorable benefit / risk ratio of the drug under investigation Eli. S Lec. No. 6 5

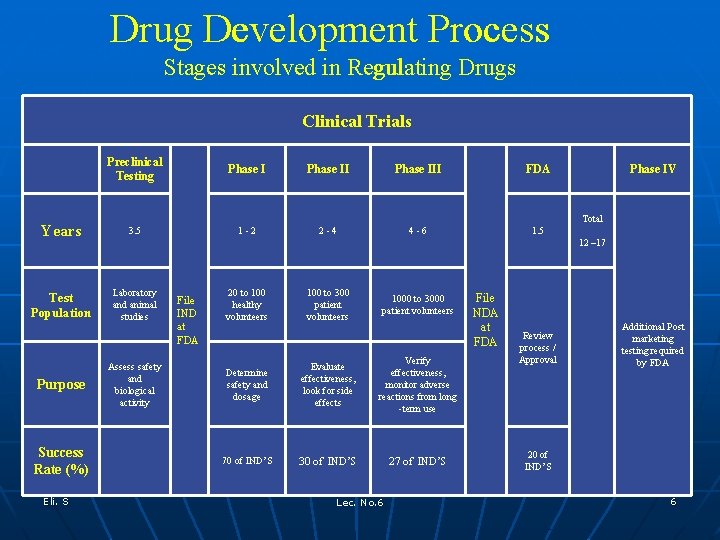
Drug Development Process Basic Disciplines of Drug Development Stages involved in Regulating Drugs Clinical Trials Preclinical Testing Phase III FDA Years 3. 5 1 -2 2 -4 4 -6 1. 5 Test Population Laboratory and animal studies Purpose Success Rate (%) Eli. S Phase IV Total 12 – 17 Assess safety and biological activity File IND at FDA 20 to 100 healthy volunteers 100 to 300 patient volunteers 1000 to 3000 patient volunteers Determine safety and dosage Evaluate effectiveness, look for side effects Verify effectiveness, monitor adverse reactions from long -term use 70 of IND’S 30 of IND’S 27 of IND’S Lec. No. 6 File NDA at FDA Review process / Approval Additional Post marketing testing required by FDA 20 of IND’S 6

Role of Laboratories in Medicine n n n Medical Testing Laboratory patient care clinical drug trial Research Laboratory discovery & development of new drugs/therapy fundamental research/mechanisms of diseases Manufacturing post clinical trials, bulk drugs, cell-based therapy, plasma products, medical device Eli. S Lec. No. 6 7

REFERANCE Good Laboratory Practice by Ainoon Othman Department of Pathology Faculty of Medicine, UKM Eli. S Lec. No. 6 8