Drug Design Functional groups Pharmacological Activity Structure Mechanism


![Cyclopentolate - tertiary amine, p. Ka ca. 10 p. H=p. Ka; [acid]= [base] p. Cyclopentolate - tertiary amine, p. Ka ca. 10 p. H=p. Ka; [acid]= [base] p.](https://slidetodoc.com/presentation_image_h2/b7531015c1436b619abdb9adadd05bf7/image-6.jpg)








- Slides: 24

Drug Design: Functional groups / Pharmacological Activity Structure - Mechanism of action (Interaction with target) Structure - Physiochemical properties (Bioavailability etc) • • • Acid / base properties Water solubility Partition coefficient (Crystal structure) Stereochemistry ADME Absorbtion. Distribution, Metabolism, Excretion (ADMET, ADMEtox) KJM 5230 -H 06

Structure - Mechanism of action KJM 5230 -H 06

Structure - Mechanism of action SAR: Structure Activity Relationships Acetylcholine agonists: Small N-quartenary compds. Acetylcholine antagonists: Larger N-quartenary compds. KJM 5230 -H 06

Active compound identified Target? Target identified Ligand? KJM 5230 -H 06

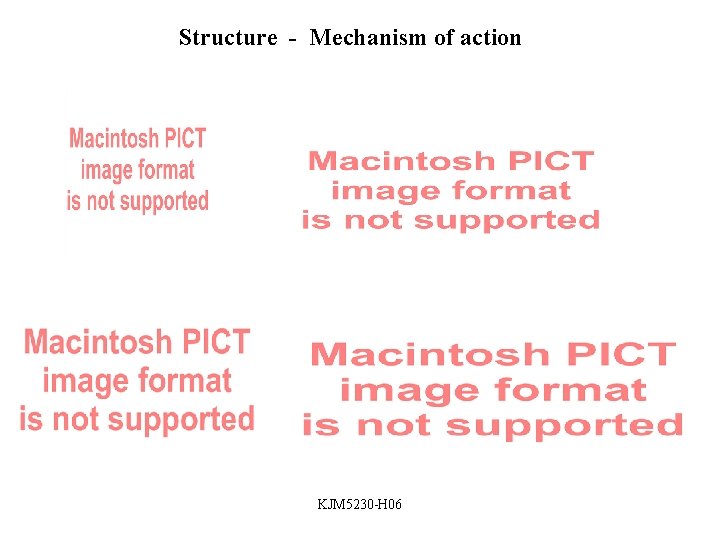
Structure - Physiochemical properties • Acid / base properties • • Water solubility Partition coefficient (Crystal structure) Stereochemistry Human body: ca 75% water p. H blood ca 7. 4 (physiolog. p. H) p. H stomach 1 - 3. 5 p. H duodenum ca. 4 p. H urine ca. 6 Identification of acidic / basic functional groups p. Ka determines degree of ionization different places in the body KJM 5230 -H 06
![Cyclopentolate tertiary amine p Ka ca 10 p Hp Ka acid base p Cyclopentolate - tertiary amine, p. Ka ca. 10 p. H=p. Ka; [acid]= [base] p.](https://slidetodoc.com/presentation_image_h2/b7531015c1436b619abdb9adadd05bf7/image-6.jpg)
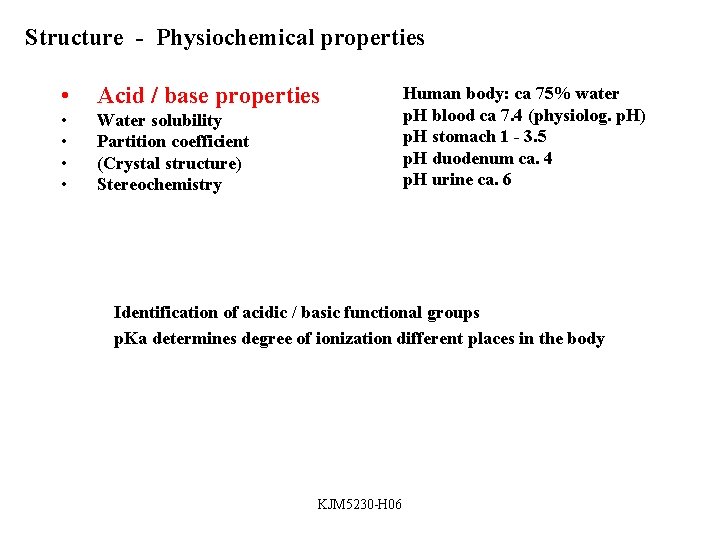
Cyclopentolate - tertiary amine, p. Ka ca. 10 p. H=p. Ka; [acid]= [base] p. H<p. Ka; acid form dominates p. H>p. Ka; basic form dominates = active form KJM 5230 -H 06

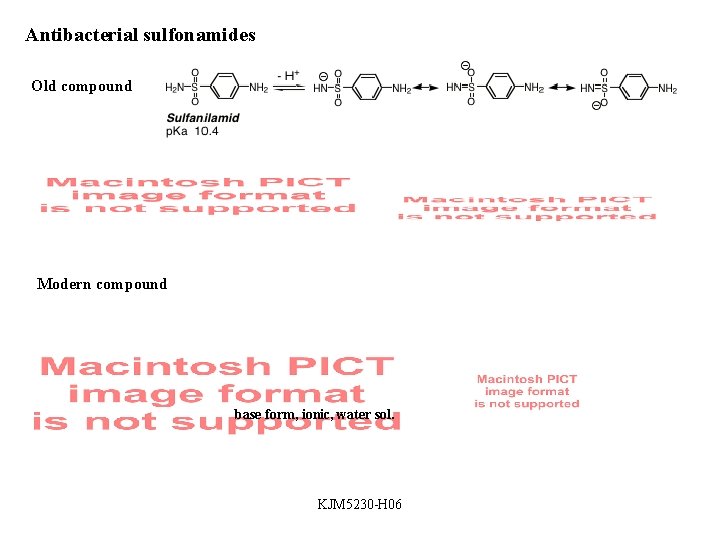
Antibacterial sulfonamides Old compound Modern compound base form, ionic, water sol. KJM 5230 -H 06

Structure - Physiochemical properties • Acid / base properties • Water solubility Ionisation • • • Partition coefficient (Crystal structure) Stereochemistry Hydrogen bonds -permanent charge -acid / base properties Salts between weak organic acids and weak organic bases does not dissolve well in water KJM 5230 -H 06

The more H-bonds possible - the more water sol. KJM 5230 -H 06

Prediction of water solubility - Empirical Water solubilization of functional groups Ex. monofuctional comp. methanol - pentanol/hexanol are solubile Charge: 1 charge - 20 -30 C (solubile: >10 mg/m. L) KJM 5230 -H 06

Water solubilization of functional groups Ex. polyfunctional comp. Charge: 1 charge - 20 -30 C KJM 5230 -H 06

Structure - Physiochemical properties • • Acid / base properties Water solubility • Partition coefficient • • (Crystal structure) Stereochemistry log. P P: Partition coefficient between n-octanol and water Experimental: Mlog. P or log. Pmeas log. P Rt (HPLC, TLC reverse phease) Calcd: Clog. P p-value: hydrophilic - lipophilic value KJM 5230 -H 06 Clog. P (Sci. Finder): 2. 69

Absorbtion of Bioactive Compounds Absorbtion from GI tract KJM 5230 -H 06

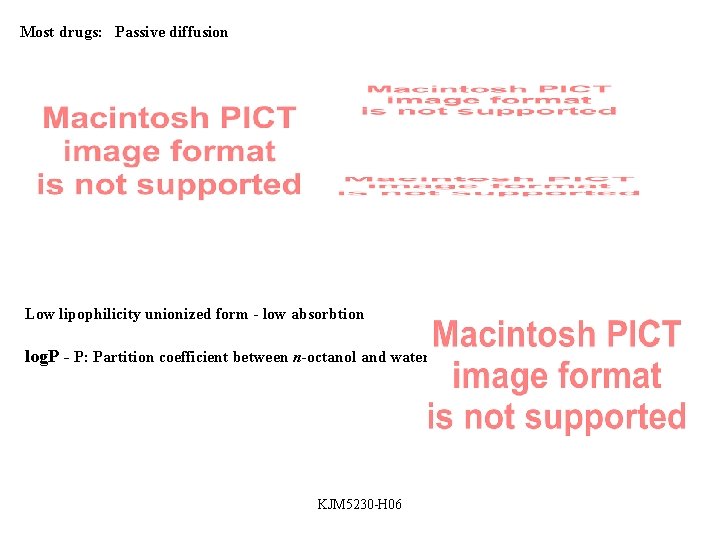
Most drugs: Passive diffusion Low lipophilicity unionized form - low absorbtion log. P - P: Partition coefficient between n-octanol and water KJM 5230 -H 06

Crossing the membrane Passive transport / diffusion Rate Conc. absortion site (1. order kinetics) % Drug absorbed lipophilicity Size of molecule Certain ionic compounds may go thru as ion-pair KJM 5230 -H 06

Active transport / Carrier mediated transport • Less common • Structural recemblanse with for instance nutritional compound • Transport against conc. gradient • Mechanism saturated at high conc. • Competition for carrier molecules, compounds with structural resemblance KJM 5230 -H 06

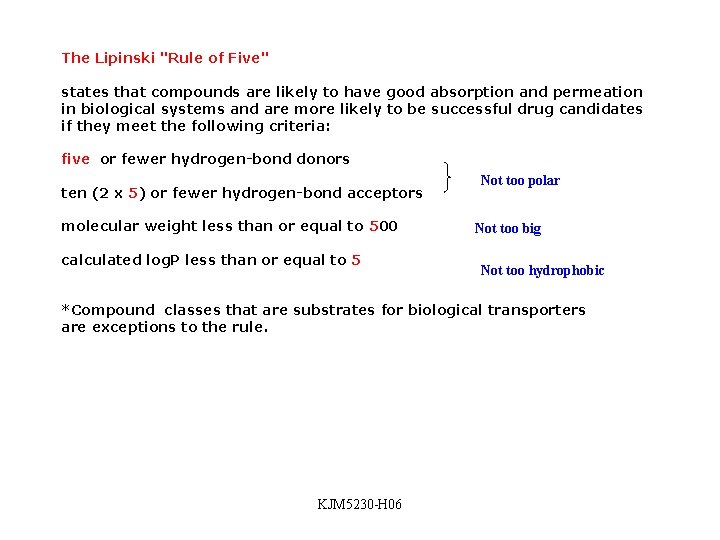
The Lipinski "Rule of Five" states that compounds are likely to have good absorption and permeation in biological systems and are more likely to be successful drug candidates if they meet the following criteria: five or fewer hydrogen-bond donors ten (2 x 5) or fewer hydrogen-bond acceptors molecular weight less than or equal to 500 calculated log. P less than or equal to 5 Not too polar Not too big Not too hydrophobic *Compound classes that are substrates for biological transporters are exceptions to the rule. KJM 5230 -H 06

Structure - Physiochemical properties • • Acid / base properties Water solubility Partition coefficient (Crystal structure) • Stereochemistry Biomolecules (reseptors, enzymes): Asymmetric Enantiomers may behave differently: • Absorbtion (membrane selectivity) • Metabolism • Binding to other reseptors than target (loss, side effects) • Binding to target reseptor

Restricted rotation - optically active rotamers KJM 5230 -H 06

• Screening/Design/Serendipity/Natural products • Lead compound • Structure Optimisation • Actual Drug Refinement of lead structure: • Determining pharmacophore • Functional group modification Pharmacophore: The part of the molecule that contains the functional groups that actually binds to the reseptor KJM 5230 -H 06

Antimycobacterials KJM 5230 -H 06

Improvement of lead by functional group modification • Activity • Toxicity • Bioavailability • Metabolism Isosters: Functional groups that results in approx. the same properties Steric and electronic similarities KJM 5230 -H 06

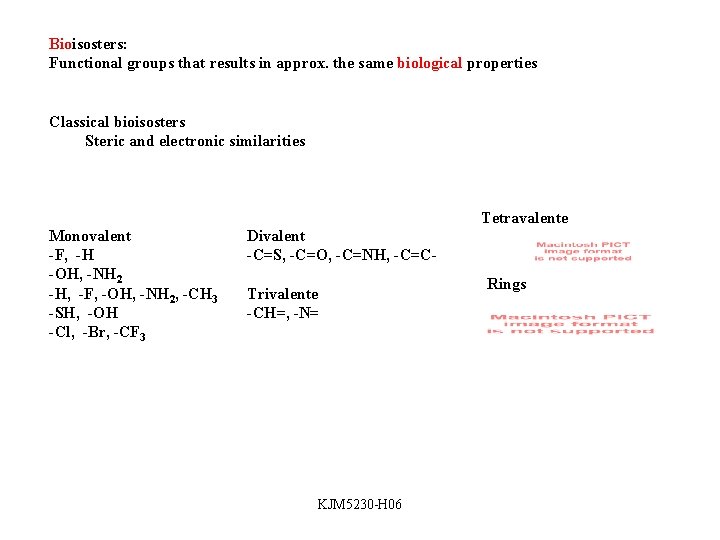
Bioisosters: Functional groups that results in approx. the same biological properties Classical bioisosters Steric and electronic similarities Tetravalente Monovalent -F, -H -OH, -NH 2 -H, -F, -OH, -NH 2, -CH 3 -SH, -OH -Cl, -Br, -CF 3 Divalent -C=S, -C=O, -C=NH, -C=CTrivalente -CH=, -N= KJM 5230 -H 06 Rings

Non-classical bioisosters Not strong steric or electronic similarities KJM 5230 -H 06