Drip Irrigation vs Regular Irrigation Do Now First






- Slides: 23

Drip Irrigation vs. Regular Irrigation

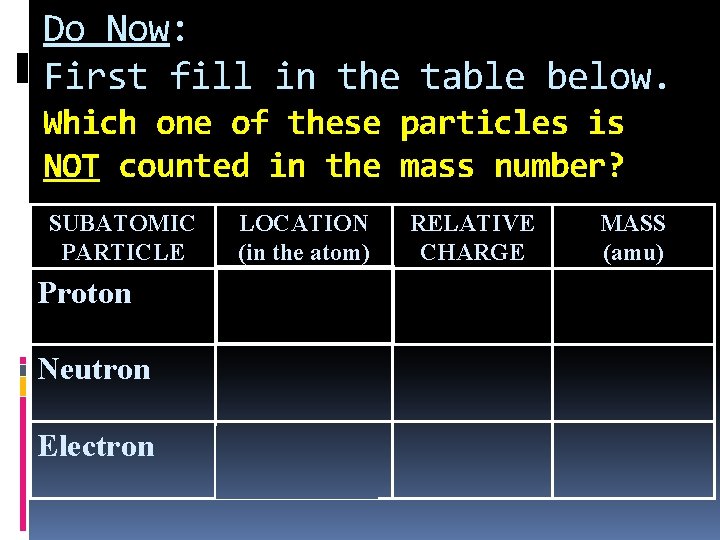
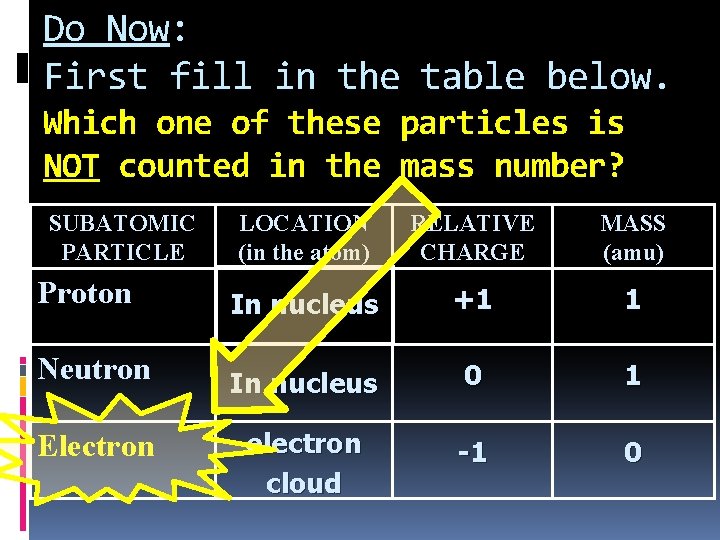
Do Now: First fill in the table below. Which one of these particles is NOT counted in the mass number? SUBATOMIC PARTICLE LOCATION (in the atom) RELATIVE CHARGE MASS (amu) Proton In nucleus +1 1 Neutron In nucleus 0 1 Electron electron cloud -1 0

Do Now: First fill in the table below. Which one of these particles is NOT counted in the mass number? SUBATOMIC PARTICLE LOCATION (in the atom) RELATIVE CHARGE MASS (amu) Proton In nucleus +1 1 Neutron In nucleus 0 1 Electron electron cloud -1 0

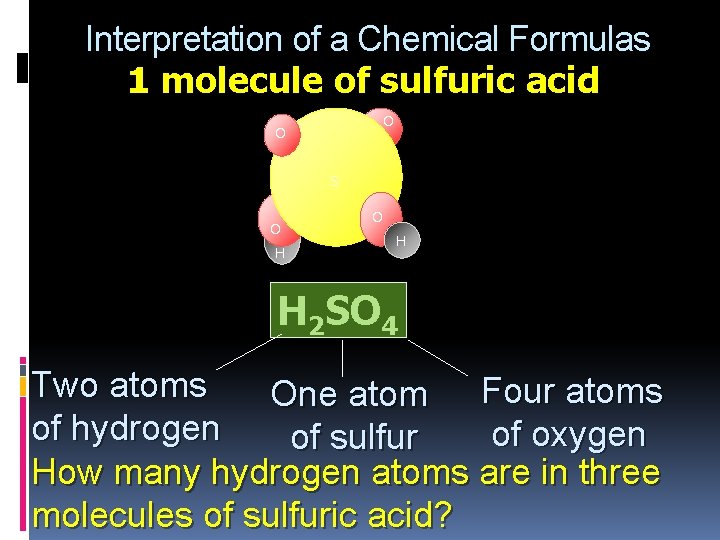
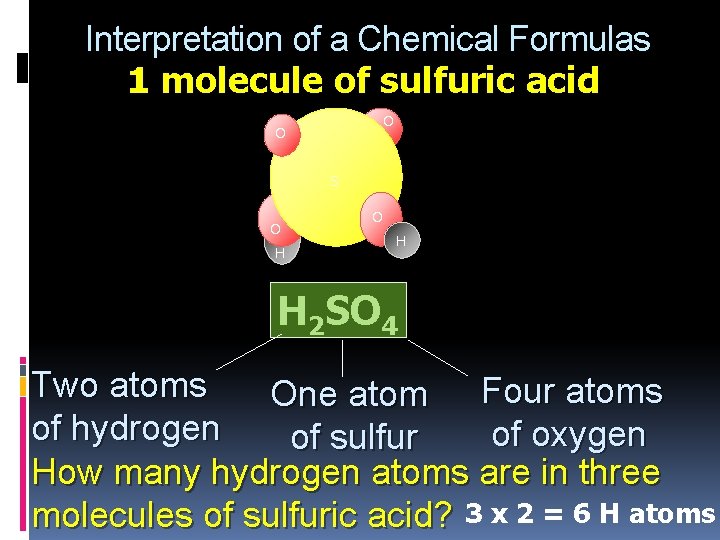
Interpretation of a Chemical Formulas 1 molecule of sulfuric acid O O S O H H 2 SO 4 Two atoms One atom Four atoms of hydrogen of oxygen of sulfur How many hydrogen atoms are in three molecules of sulfuric acid? 3 x 2 = 6 H atoms

Interpretation of a Chemical Formulas 1 molecule of sulfuric acid O O S O H H 2 SO 4 Two atoms One atom Four atoms of hydrogen of oxygen of sulfur How many hydrogen atoms are in three molecules of sulfuric acid? 3 x 2 = 6 H atoms

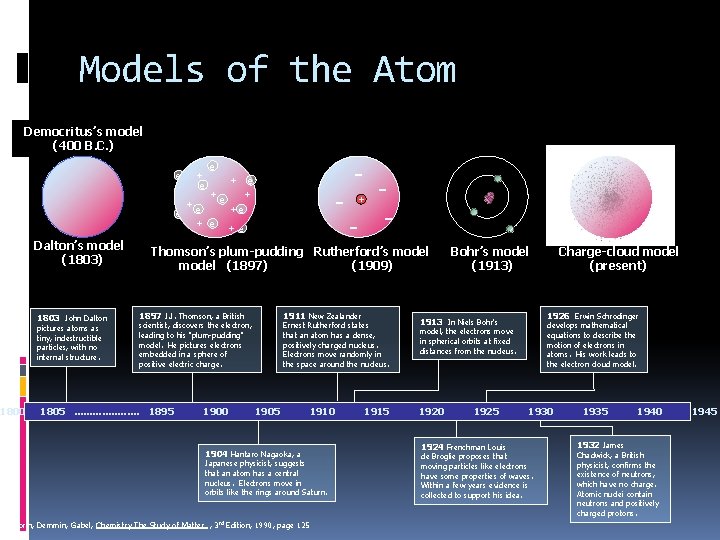
Models of the Atom Democritus’s model (400 B. C. ) e + e +e +e e + e Dalton’s model (1803) 1803 John Dalton pictures atoms as tiny, indestructible particles, with no internal structure. 1800 - - - + Thomson’s plum-pudding Rutherford’s model (1897) (1909) 1897 J. J. Thomson, a British 1911 New Zealander scientist, discovers the electron, leading to his "plum-pudding" model. He pictures electrons embedded in a sphere of positive electric charge. 1805. . . . . 1895 1900 Ernest Rutherford states that an atom has a dense, positively charged nucleus. Electrons move randomly in the space around the nucleus. 1905 1910 1904 Hantaro Nagaoka, a Japanese physicist, suggests that an atom has a central nucleus. Electrons move in orbits like the rings around Saturn. Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 125 1915 Bohr’s model (1913) 1926 Erwin Schrodinger 1913 In Niels Bohr's develops mathematical equations to describe the motion of electrons in atoms. His work leads to the electron cloud model, the electrons move in spherical orbits at fixed distances from the nucleus. 1920 1925 1924 Frenchman Louis Charge-cloud model (present) 1930 de Broglie proposes that moving particles like electrons have some properties of waves. Within a few years evidence is collected to support his idea. 1935 1932 James 1940 Chadwick, a British physicist, confirms the existence of neutrons, which have no charge. Atomic nuclei contain neutrons and positively charged protons. 1945

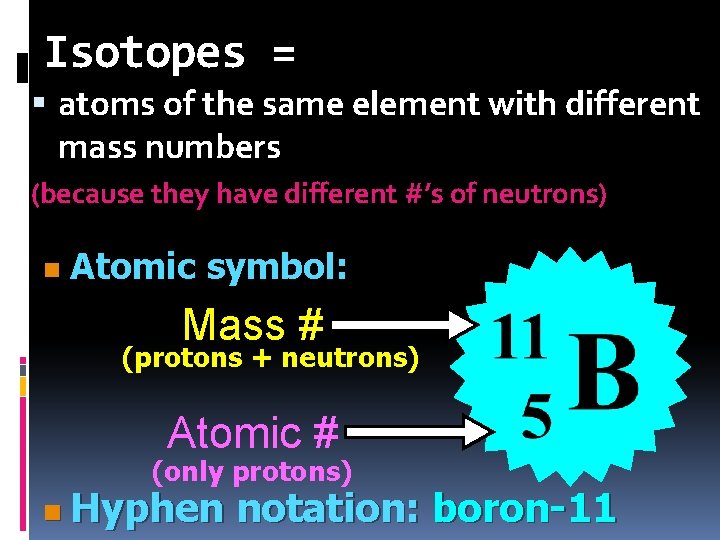
Isotopes = atoms of the same element with different mass numbers (because they have different #’s of neutrons) n Atomic symbol: Mass # (protons + neutrons) Atomic # (only protons) n Hyphen notation: boron-11

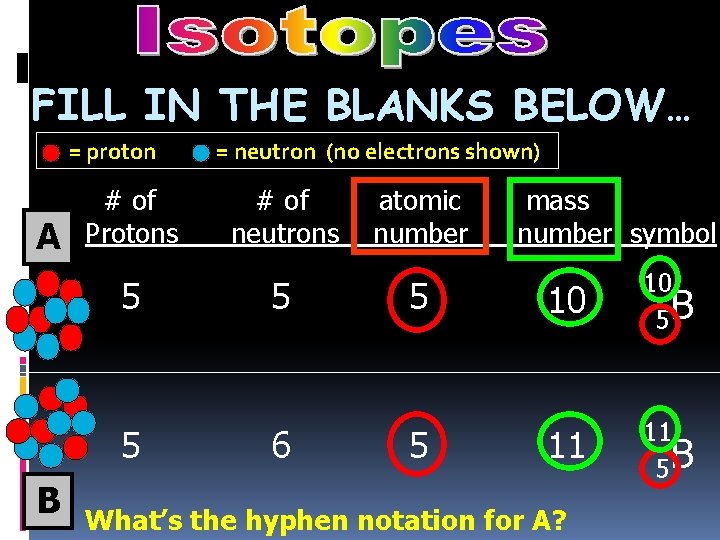
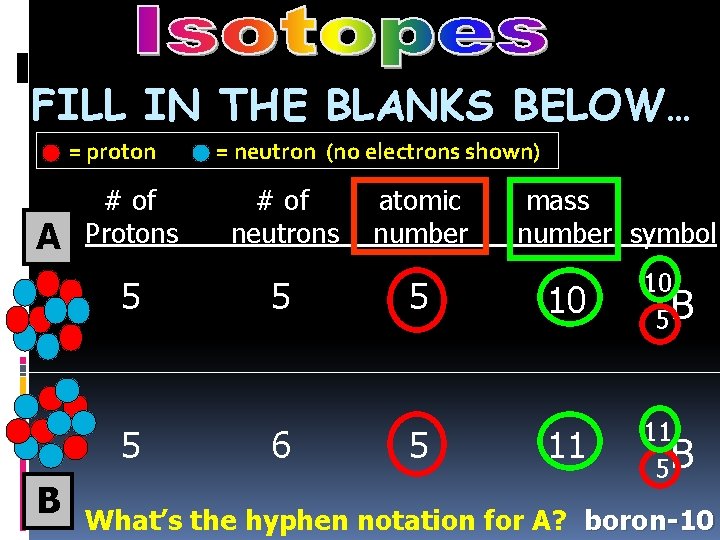
FILL IN THE BLANKS BELOW… = proton A B # of Protons = neutron (no electrons shown) # of neutrons atomic number mass number symbol

FILL IN THE BLANKS BELOW… = proton A # of Protons 5 5 B = neutron (no electrons shown) # of neutrons 5 6 atomic number 5 5 mass number symbol 10 10 5 B 11 11 5 B What’s the hyphen notation for A? boron-10

FILL IN THE BLANKS BELOW… = proton A # of Protons 5 5 B = neutron (no electrons shown) # of neutrons 5 6 atomic number 5 5 mass number symbol 10 10 5 B 11 11 5 B What’s the hyphen notation for A? boron-10

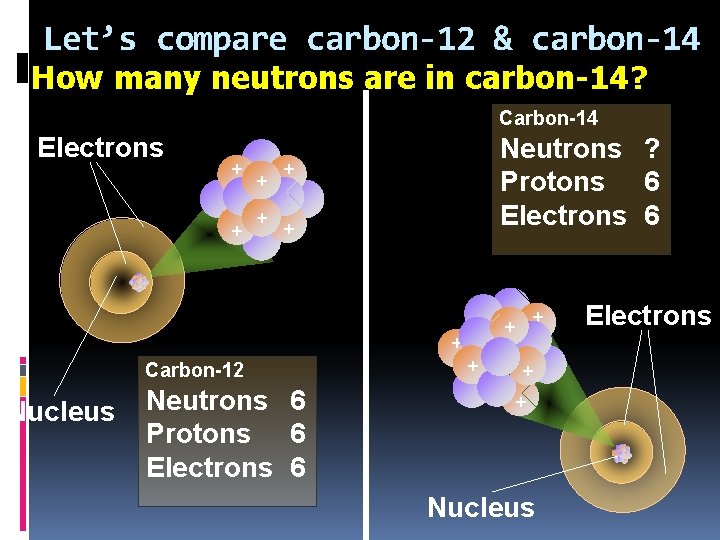
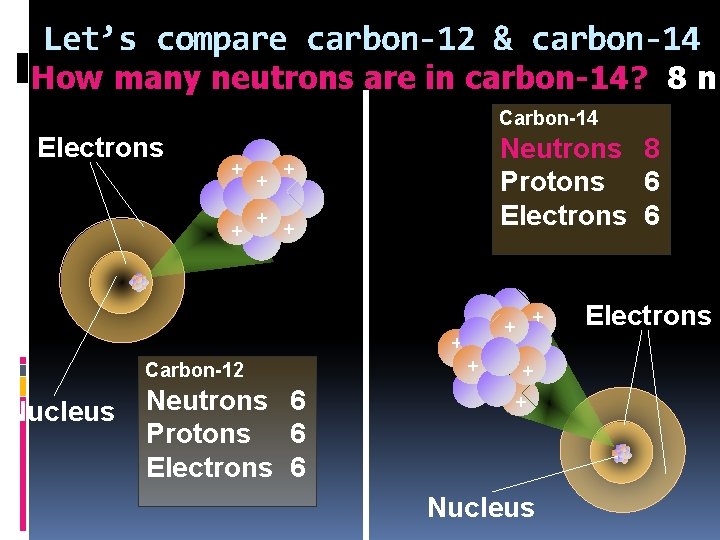
Let’s compare carbon-12 & carbon-14 How many neutrons are in carbon-14? 8 n Carbon-14 Electrons + + Neutrons ? Protons 6 Electrons 6 + + + Carbon-12 Nucleus Neutrons 6 Protons 6 Electrons 6 + + + Nucleus Electrons

Let’s compare carbon-12 & carbon-14 How many neutrons are in carbon-14? 8 n Carbon-14 Electrons + + Neutrons 8 Protons 6 Electrons 6 + + + Carbon-12 Nucleus Neutrons 6 Protons 6 Electrons 6 + + + Nucleus Electrons

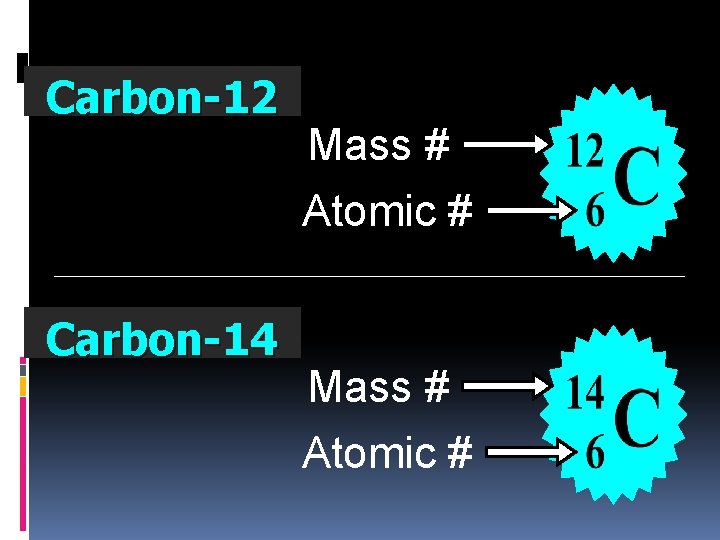
Carbon-12 Mass # Atomic # Carbon-14 Mass # Atomic #

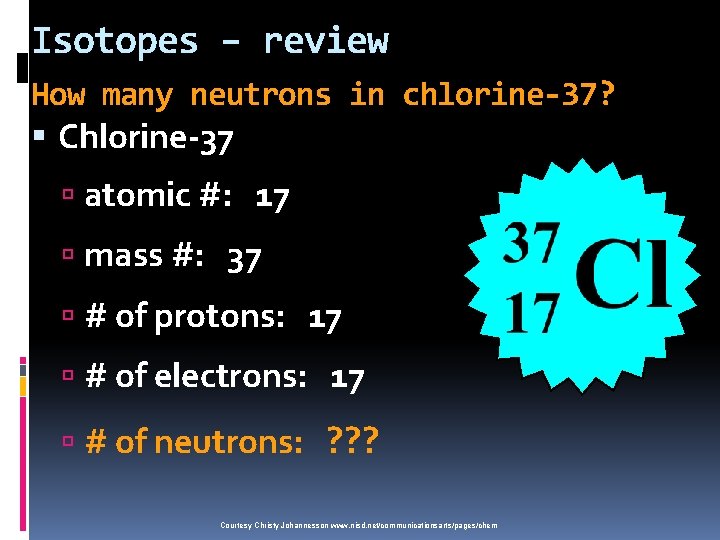
Isotopes – review How many neutrons in chlorine-37? Chlorine-37 atomic #: 17 mass #: 37 # of protons: 17 # of electrons: 17 # of neutrons: ? ? ? Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 20 n

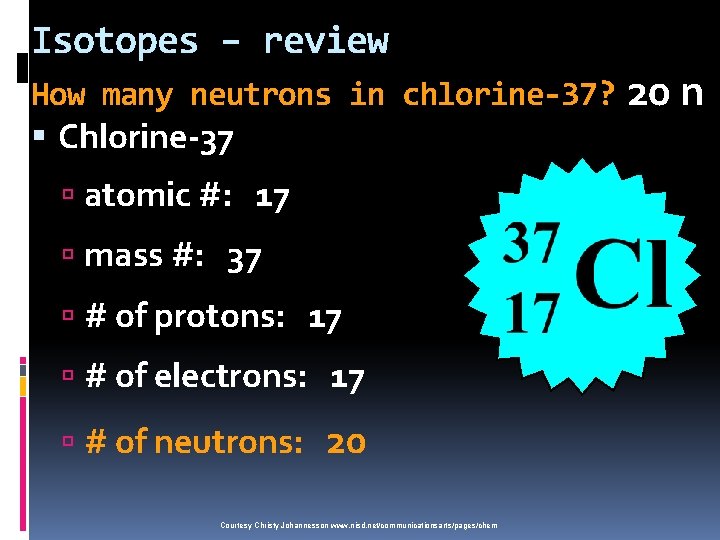
Isotopes – review How many neutrons in chlorine-37? Chlorine-37 atomic #: 17 mass #: 37 # of protons: 17 # of electrons: 17 # of neutrons: 20 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 20 n

IONS Ions are atoms that have lost or gained electrons An atom that loses an electron becomes a positive ion (CATION) An atom that gains an electron becomes a negative ion (ANION)

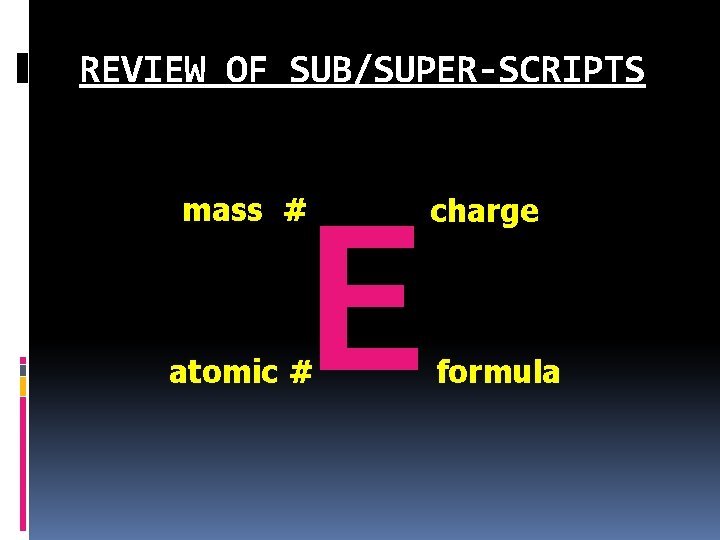
REVIEW OF SUB/SUPER-SCRIPTS E mass # atomic # charge formula

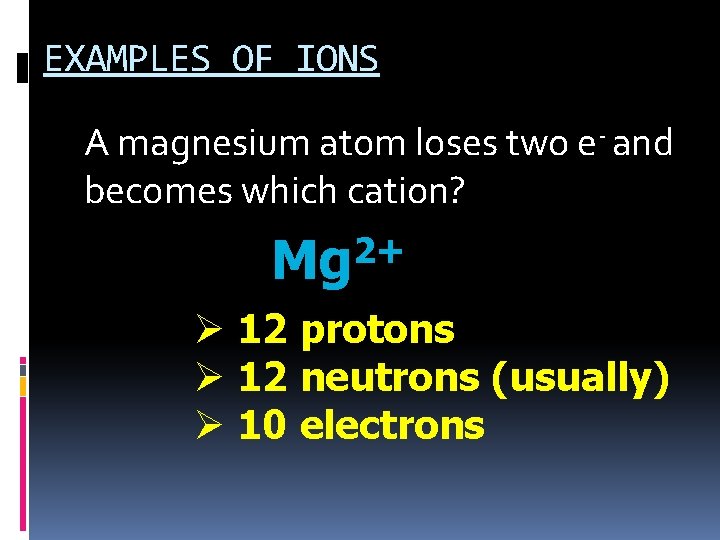
EXAMPLES OF IONS 1. A magnesium atom loses two e- and becomes which cation? 2+ Mg Ø 12 protons Ø 12 neutrons (usually) Ø 12 electrons

EXAMPLES OF IONS A magnesium atom loses two e- and becomes which cation? 2+ Mg Ø 12 protons Ø 12 neutrons (usually) Ø 10 electrons

EXAMPLES OF IONS A chlorine atom gains one e- and becomes which anion? 1 Cl Ø 17 protons Ø 18 neutrons (usually) Ø 17 electrons

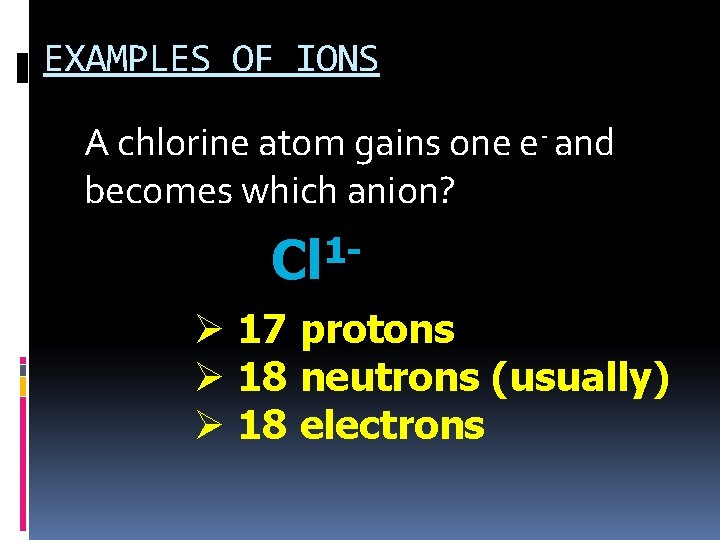
EXAMPLES OF IONS A chlorine atom gains one e- and becomes which anion? 1 Cl Ø 17 protons Ø 18 neutrons (usually) Ø 18 electrons

EXAMPLES OF IONS If magnesium and chloride bond together, what’s the formula of the compound they form? Mg. Cl 2 magnesium chloride… more on this next chapter

EXAMPLES OF IONS If magnesium and chloride bond together, what’s the formula of the compound they form? Mg. Cl 2 magnesium chloride… more on this next chapter