DRINKING WATER CLINIC INTERPRETATION MEETING EXTENSION AGENT COUNTY































- Slides: 37

DRINKING WATER CLINIC INTERPRETATION MEETING EXTENSION AGENT, COUNTY ERIN LING AND BRIAN BENHAM VIRGINIA TECH BIOLOGICAL SYSTEMS ENGINEERING VIRGINIA COOPERATIVE EXTENSION

PRIVATE WATER SUPPLIES IN VIRGINIA 1. 6 million Virginians rely on wells, springs or cisterns (21% of the population) In the U. S. municipal water supplies are regulated by the EPA under the Safe Drinking Water Act; private supplies are not! Homeowners relying on private water supplies: Are responsible for all aspects of water system management May lack knowledge and resources to effectively manage Usually don’t worry about maintenance until problems arise Groundwater is a shared resource – our actions can affect others’ water supplies too! 2

HOW DOES WATER MOVE TO MY WELL? (BEDROCK/DRILLED WELL) Well casing extends through loose “overburden” and into the bedrock, where an “open” borehole continues underground Groundwater moves through fractures, or cracks in the bedrock Water can come from many different directions, depths, and sources into one well It can take water hours, days, or years to move through to bedrock Water can come from any fractures that intersect the open borehole 3

HOW DOES WATER MOVE TO MY WELL? (SCREENED WELL) In drilled or bored wells in sandy aquifers, groundwater fills up the pore spaces between grains of sediment or sand In shallow wells, water moves relatively quickly from the surface down into the water table; with deeper wells, it takes more time. There a large range of depths of wells reaching aquifers at varying levels 4

PROPER WELL LOCATION AND CONSTRUCTION Not in an area that receives runoff Ground slopes away from well Drilled well 12” Well casing at least 12” above ground Grout seal around casing (have checked by a well driller) Sanitary well cap (drilled well) or sealed concrete cover (bored well) 12” Bored/dug well Photo credits: SAIF Water Wells ; Penn State University At least 50 -100’ and upslope from contamination sources

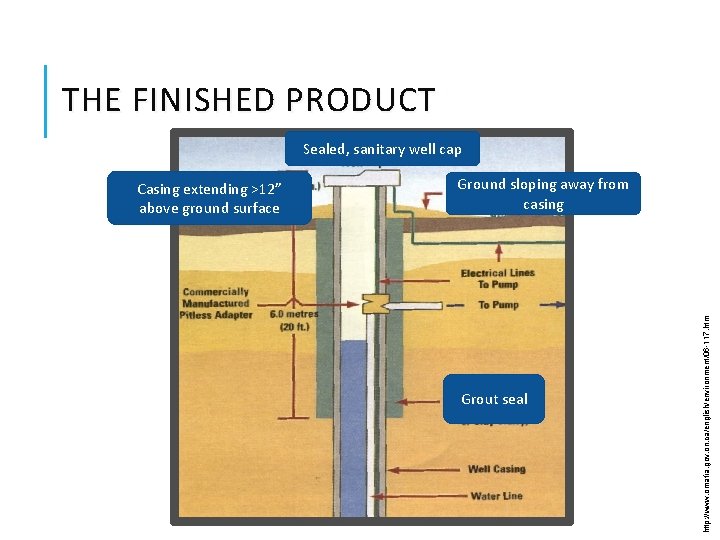
THE FINISHED PRODUCT Sealed, sanitary well cap Ground sloping away from casing Grout seal http: //www. omafra. gov. on. ca/english/environment/06 -117. htm Casing extending >12” above ground surface

WELL MAINTENANCE TIPS Do not use fertilizers, pesticides, oil, or paint near well Keep area around well clean and accessible Keep careful records original contract, water test results and any maintenance or repair information Every year: Conduct thorough visual inspection of well Check cap for cracks, wear and tear, tightness Test for coliform bacteria (at least)! Every 1 -3 years have well inspected by a licensed well driller – see Well. Check page and providers!

PRIVATE WATER SUPPLY REGULATIONS Virginia Private Well Regulations • Specify application, inspection and construction requirements • No requirements for maintenance or water testing after construction of well – responsibility of the owner! EPA National Drinking Water Standards • Apply to PUBLIC systems • Primary (health) and Secondary (nuisance) • Can be used as guidance for private systems to know “how much is too much”

EPA SAFE DRINKING WATER ACT Primary Standards Secondary Standards Also called Maximum Contaminant Level (MCL) Also called SMCL (Secondary) or RMCL (Recommended) Cause health problems Cause aesthetic problems: Enforced for municipal systems • Includes specific chemicals and pesticides Over 80 contaminants, including • • • Lead Coliform and E. coli bacteria Copper Arsenic Nitrate Staining • Taste • Odor Many naturally occur in ground water About 15 contaminants, including: Iron • Sulfate • Manganese • Hardness • 9

TESTING WATER QUALITY Why test? • Protect family’s health and safety • Many contaminants undetectable by human senses • Preventive measures often more effective and less expensive • Possible legal protection in case of changes to water due to nearby impacts When to test? • Routine tests every 1 -3 years • Pregnant woman or infant in the home • Recurring gastrointestinal illness (guests too!) • Change in taste, appearance, or odor of water • Any time services or repairs are done (well cap opened/pump pulled up)

WHAT SHOULD I TEST FOR? Every year test for coliform bacteria and E. coli • Simple, relatively inexpensive test • Indicates possible contamination from human or animal waste About every three years test for other contaminants based on nearby land uses and condition of water • p. H • Total dissolved solids

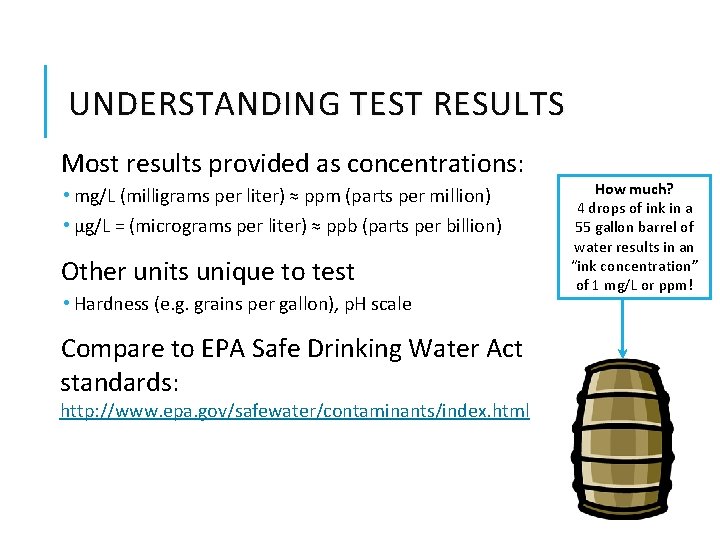
UNDERSTANDING TEST RESULTS Most results provided as concentrations: • mg/L (milligrams per liter) ≈ ppm (parts per million) • µg/L = (micrograms per liter) ≈ ppb (parts per billion) Other units unique to test • Hardness (e. g. grains per gallon), p. H scale Compare to EPA Safe Drinking Water Act standards: http: //www. epa. gov/safewater/contaminants/index. html How much? 4 drops of ink in a 55 gallon barrel of water results in an “ink concentration” of 1 mg/L or ppm!

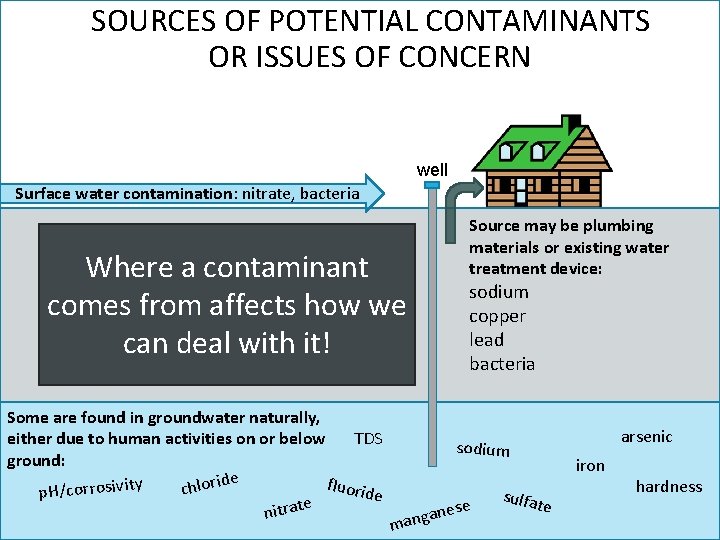
SOURCES OF POTENTIAL CONTAMINANTS OR ISSUES OF CONCERN well Surface water contamination: nitrate, bacteria Where a contaminant comes from affects how we can deal with it! Some are found in groundwater naturally, TDS either due to human activities on or below ground: ide r fluorid y it o l iv s o h r r o c c p. H/ e e t a r t ni Source may be plumbing materials or existing water treatment device: sodium copper lead bacteria arsenic sodium ese n a g man sulfat iron e hardness

OPTIONS FOR PROBLEM WATER 1. If possible, control the source of pollution • Divert runoff, maintain septic system 2. Improve maintenance of water system • Install sanitary well cap, slope the ground 3. Treat the water to reduce contaminant concentration • Match the treatment option to the pollutant • Consult a professional 4. Develop a new source of water • Deeper well, develop spring, connect to public water http: //static. howstuffworks. com/gif/septic-tank-cleaning-1. jpg, http: //www. shipewelldrilling. com/Pictures/well_drilling_rig. jpg, http: //www. clearflow. ca/REVERSE_OSMOSIS 2. jpg

TREATMENT CONSIDERATIONS No treatment device can remove all contaminants Each device has tradeoffs; be sure to explore ALL of your options Always have water tested by a third-party certified lab Certifications to look for: National Sanitation Foundation (NSF) – certifies DEVICES Water Quality Association (WQA) – trains WATER TREATMENT SPECIALISTS and certifies DEVICES Point of Use (POU) vs. Point of Entry (POE) If it sounds too good to be true, it probably is (e. g. , magnets, electronic charge, magic) Consider: upfront cost, maintenance requirements and warranty

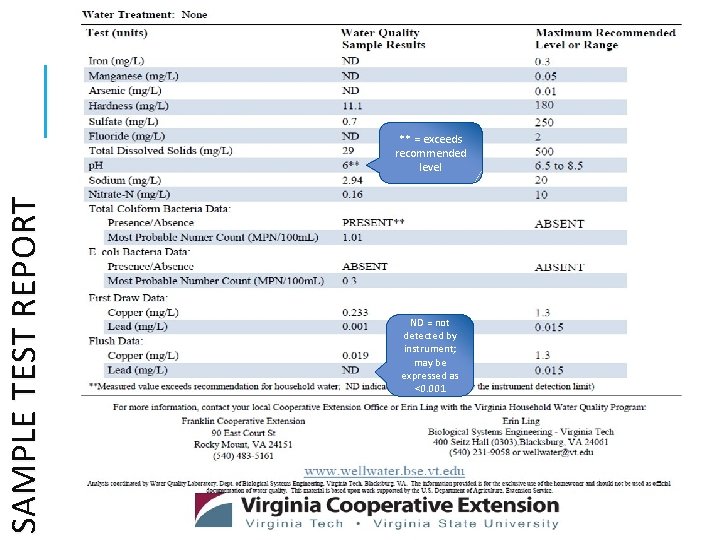
SAMPLE TEST REPORT ** = exceeds recommended level ND = not detected by instrument; may be expressed as <0. 001

COLIFORM BACTERIA Coliform bacteria is an indicator organism • Present in soil, from animals/humans, common in environment • Means disease-causing bacteria may also be present • Water recently on the surface may be reaching well water Public standard is 0, or absent (in municipal supplies) If present, lab will analyze for E. coli bacteria http: //support. cleanwaterstore. com/blog/sources-of-coliform-bacteriacontamination-in-home-well-water/ • Generally harmless in itself

IF COLIFORM BACTERIA ARE PRESENT Don’t panic! • Casing intact and extends above ground at least 12”? • Sealed, sanitary well cap is installed and secure? • Ground slopes away from well? • Has driller checked for grout seal? Shock chlorination; retest after 2 -4 weeks Long term treatment options: UV light, ozonation, continuous chlorination http: //support. cleanwaterstore. com/blog/sources-of-coliform-bacteriacontamination-in-home-well-water/ Check well for pathways surface water can enter well

IF E. COLI BACTERIA ARE PRESENT • Take immediate steps to address • Boil water or use another source of water for drinking or cooking Check well for pathways surface water can enter well Shock chlorinate and retest after 2 -4 weeks Long-term treatment options: UV light, ozonation, continuous disinfection http: //www. kimicontrol. com/microorg/escherichia_coli. jpg More serious result: human or animal waste is entering water supply

BACTERIA MPN How much? Colilert ™ is added to the water, which is poured into a tray and sealed. Yellow = coliform present and fluorescing under ultraviolet light = E. coli present. If bacteria are present, you will see a number under the word PRESENT. MPN = Most Probable Number (statistical estimate of the number of bacteria per 100 m. L water) Number (of coliform and E. Coli) ranges from 1 to >2419. ABSENT: MPN will be ND (not detected) TNTC = Too Numerous To Count = >2419 MPN Still want to see both types of bacteria ABSENT Gives people an idea of the extent of the problem for both total coliform and E. coli.

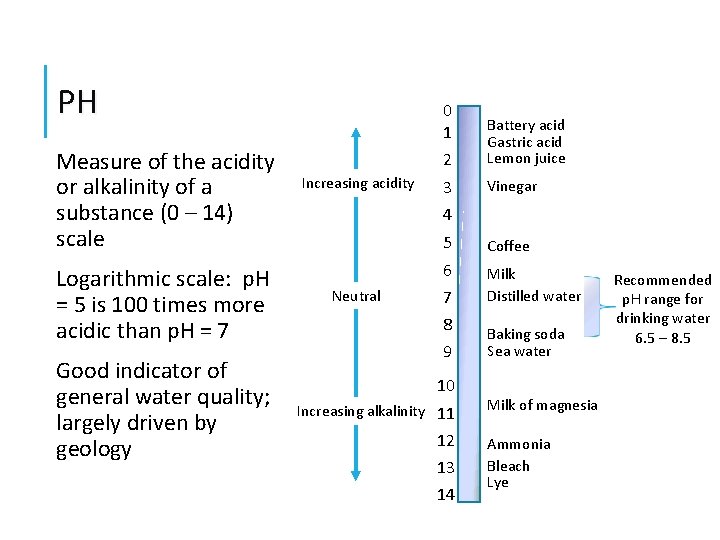
PH Measure of the acidity or alkalinity of a substance (0 – 14) scale Logarithmic scale: p. H = 5 is 100 times more acidic than p. H = 7 Good indicator of general water quality; largely driven by geology Increasing acidity 0 1 2 Battery acid Gastric acid Lemon juice 3 Vinegar 4 Neutral 5 Coffee 6 Milk Distilled water 7 8 9 10 Increasing alkalinity 11 12 13 14 Baking soda Sea water Milk of magnesia Ammonia Bleach Lye Recommended p. H range for drinking water 6. 5 – 8. 5

CORROSIVE WATER Water is “aggressive”; corrodes metal plumbing Dissolves metals present into drinking water Causes pitting and pinhole leaks Commonly caused by low p. H; other contributing factors include alkalinity, temperature, TDS levels EPA recommends drinking water be non-corrosive; metals like lead and copper can be a health concern Long-term treatment: acid neutralizing filter or soda ash injection (depending on p. H) http: //www. bushman. cc/photos/Copper_Water_Pipe_Corrosion. jpg; Reduces length of appliance life (e. g. water heaters)

METALS OF CONCERN: LEAD Lead • Linked to many serious health effects, especially in children and infants • Developmental, neurological, reproductive and renal problems • Cumulative poison; small amounts accumulate in body over time Lead MCL Goal is 0 mg/L with a health action level of 0. 015 mg/L Sources include: • Lead solder in homes built prior to 1986 • “Lead-free” brass fittings and fixtures (<8% lead until January 2014, when the allowed level was lowered to 0. 25%) • Some galvanized steel components in wells

ADDRESSING LEAD IN WATER There is no safe level of lead. Even if below 0. 015 ppm, take action, especially if children/pregnant women are drinking the water Discuss test results with your physician Compare first draw and flushed • Metals may be highest with corrosive water and contact time with pipes. • Flushing pipes may address problem, if flushed lead level is considerably lower.

ADDRESSING LEAD IN WATER There is no safe level of lead. Even if below 0. 015 ppm, take action, especially if children/pregnant women are drinking the water Treatment options: • Activated carbon filter (e. g. Brita or Pur) MAKE SURE IT IS LABELED TO REMOVE LEAD AND CHANGE AS DIRECTED Acid neutralizing filter can address corrosivity of water if p. H < 6. 5; however, corrosivity can be caused by other factors as well IF LEAD IS ELEVATED IN FLUSHED SAMPLE, ACTIVATED CARBON MAY STILL BE NECESSARY • Reverse osmosis device will also remove dissolved metals Or, use another source of water known to be safe

METALS OF CONCERN: COPPER High levels can cause nausea, vomiting, stomach cramps Infants and children particularly sensitive EPA MCL is 1. 3 mg/L Nuisance effects, such as blue-green staining, noticeable at 1. 0 mg/L http: //www. gravitaexim. com/images/Lead-pipe. jpg

Nuisance - not health concern for most SMCL: Iron = 0. 3 mg/L; Mn = 0. 05 mg/L Red/brown/black staining, particles, metallic taste Tend to occur together in geology Concentrations should be ADDED to determine treatment Treatment depends on type/form of each http: //www. freedrinkingwater. com/images-water-quality/chemicals/water%20 in%20 reddish-brown. jpg IRON AND MANGANESE Ferrous/Manganous: water initially clear orange-brown or black solid particles Ferric/Manganic: solid particles visible immediately, or water has a tint Iron bacteria: not a health concern; feed on Fe and Mn, red-brown or black-brown slime Treatment: water softener, aeration/filtration, ozonation, distillation

www. goodcleanwater. com/fyi. htm; www. watersoftening. org/effects_of_hard_water. htm; HARDNESS/SCALING Hard water contains high levels of calcium and magnesium ions, dissolved from limestone and other minerals Not a health risk – nuisance • Decreased cleaning action of soaps, detergents • Scale build-up in pipes and on appliances • Reduced efficiency and lifespan of water heaters No EPA standard for public systems Treat using water softener Hardness Rating Grains per Gallon mg/L Soft Less than 1. 0 Less than 17. 1 Slightly-Moderately Hard 1. 0 -7. 0 17. 1 -120 Hard 7. 0 -10. 5 120 -180 Very Hard Over 10. 5 Over 180

TOTAL DISSOLVED SOLIDS (TDS) Water is a solvent: dissolves many compounds as it travels over and under ground TDS is a measure of all dissolved impurities Man-made sources: septic systems, runoff from agricultural or urban land, road salt, industrial sources General indicator of water quality; test at least every three years EPA SMCL is 500 mg/L Treat using distillation or reverse osmosis http: //en. wikipedia. org/wiki/Total_dissolved_solids Natural sources: limestone, salt deposits, other minerals

Varying levels occur naturally; high levels may be from man-made source Road salt storage or application, industrial waste, sewage, fertilizers or animal waste Natural sources (some bedrock and sediments) WATER SOFTENER – works by ion exchange: extracts calcium, magnesium, iron, adds sodium EPA recommendation for those on low-sodium diets: 20 mg/L Consider other sources of salt in diet and discuss with doctor Higher levels may indicate contamination – test for bacteria or other contaminants Salty taste; and may accelerate corrosion of pipes and water heaters Treat using distillation, reverse osmosis /www. cotrip. org/winterdriving/images/pic 6. jpg; /www. apswater. com/images/fleck%205600. jpg SODIUM

http: //wi. water. usgs. gov/pubs/FS-221 -95/p 2. gif NITRATE (NO 3 -N) Serious health concern for infants Methemoglobinemia or “blue baby syndrome” Nitrate nitrite during digestion and blood cannot carry oxygen MCL is 10 mg/L NO 3 -N or 45 mg/L of NO 3 If 3 -5 mg/L, do not use water for infants under 6 months Sources include fertilizer, animal manure, sewage; NO 3 dissolves and moves easily through soil Test in spring months; levels change over time BOILING INCREASES concentration of nitrates! Treatment: distillation, reverse osmosis, anion exchange 31

thepipelinefixation. blogspot. com HYDROGEN SULFIDE Colorless gas; rotten egg smell Not regulated by EPA – people can detect low levels Naturally present in shale, sandstone, near coal or oil fields Sulfur-reducing bacteria produce (not a health risk) Treatment depends on concentration, so must test Only noticeable in hot water? Bacteria could be thriving in your water heater Sulfates may be converted to H 2 S chemically in your water heater during a reaction with your magnesium corrosion control rod 32

ARSENIC Occurs naturally in some rocks; more common in groundwater supplies when water levels rise and fall frequently Used in wood preservatives, paints, pesticides, etc. Linked to many types of cancer, stomach pain, paralysis, and blindness EPA primary standard is 0. 010 mg/L Treatment: reverse osmosis, distillation

Occurs naturally; some high levels in E. Virginia groundwater Added to many public water systems for strong teeth and bones (levels 0. 8 -1. 2 mg/L); limit intake for kids under 8 Health concerns: Long term exposure: links to bone cancer Shorter term exposure: dental or skeletal fluorosis EPA MCL 4. 0 mg/L and SMCL 2. 0 mg/L Treatment (reverse osmosis) removes ALL fluoride http: //www. willamettedental. com/en_us/ALL/patients/pps/retailproducts_prettysmile. gif; http: //en. wikipedia. org/wiki/Dental_fluorosis FLUORIDE

Virginia Household Water Quality Program State program personnel: Erin Ling (wellwater@vt. edu) Brian Benham (benham@vt. edu) www. wellwater. bse. vt. edu email: wellwater@vt. edu ph: 540 -231 -9058 Local Extension Agent: 35

RESOURCES Virginia Household Water Quality Program www. wellwater. bse. vt. edu Virginia Certified Lab Listing: http: //www. wellwater. bse. vt. edu/files/Lablist 2016. pdf EPA Private Wells Site https: //www. epa. gov/privatewells National Groundwater Association Well Owner http: //www. wellowner. org/ Water Systems Council Wellcare Hotline http: //www. wellcarehotline. org/ NSF International: www. nsf. org Water Quality Association: www. wqa. org Consumer Reports or Better Business Bureau www. consumerreports. org OR www. bbb. org

END OF SLIDE SHOW. ADDITIONAL SLIDES FOLLOW IF YOU WISH TO ADD THEM.