Drinking Water Clinic Interpretation Meeting Erin Ling and
























- Slides: 38

Drinking Water Clinic Interpretation Meeting Erin Ling and Brian Benham Virginia Tech Biological Systems Engineering, Virginia Cooperative Extension

Why are we here? • Caring for your private water system • Well location, protection, and construction • Well maintenance and care • Drinking water regulations – knowing how much is too much • Water testing – what’s in your water? • Dealing with problems • Additional resources 2

Private Water Supplies in Virginia 1. 7 million Virginians rely on wells, springs or cisterns (22% of the population) In the U. S. municipal water supplies are regulated by the EPA under the Safe Drinking Water Act; private supplies are not! Homeowners relying on private water supplies: ◦ Are responsible for all aspects of water system management ◦ May lack knowledge and resources to effectively manage ◦ Usually don’t worry about maintenance until problems arise Groundwater is a shared resource – our actions can affect others’ water supplies too! 3

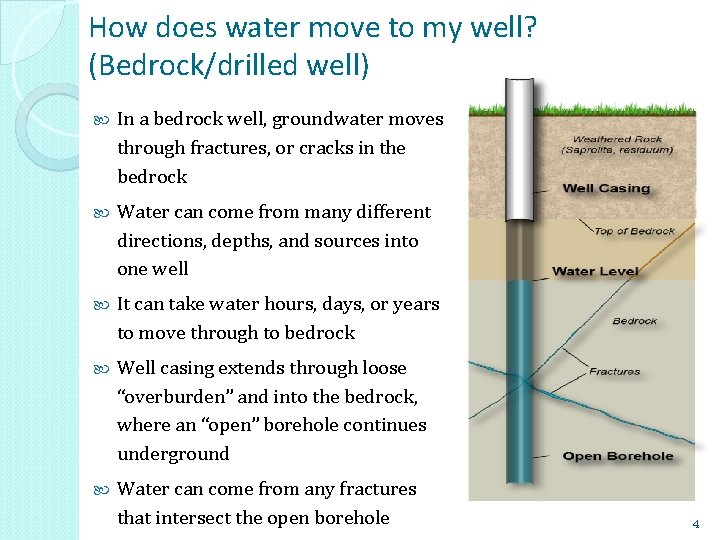
How does water move to my well? (Bedrock/drilled well) In a bedrock well, groundwater moves through fractures, or cracks in the bedrock Water can come from many different directions, depths, and sources into one well It can take water hours, days, or years to move through to bedrock Well casing extends through loose “overburden” and into the bedrock, where an “open” borehole continues underground Water can come from any fractures that intersect the open borehole 4

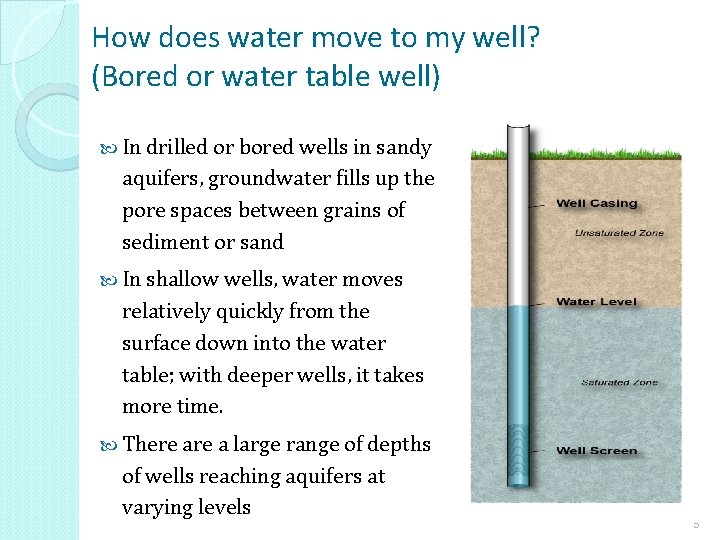
How does water move to my well? (Bored or water table well) In drilled or bored wells in sandy aquifers, groundwater fills up the pore spaces between grains of sediment or sand In shallow wells, water moves relatively quickly from the surface down into the water table; with deeper wells, it takes more time. There a large range of depths of wells reaching aquifers at varying levels 5

Photo credit: Swistock, Penn State Univ Proper well location Well should be at least: ◦ 10 feet from building foundation (50 feet if termite treated) ◦ 50 feet from road ◦ 50 feet from sewers and septic tanks ◦ 100 feet from pastures, on-lot sewage system drainfields, cesspools or barnyards Upslope from potential contamination Not in an area that receives runoff 6

Proper well construction Contract a licensed driller: ◦ Valid Class A, B or C contractor license with WWP (Water Well and Pump) classification Well casing 12” ◦ Extends 12” above ground Grouting to a minimum of 20’ Sanitary well cap or sealed concrete cover Ground slopes away from well 7 Photo credits: SAIF Water Wells ; Penn State University ◦ Minimum of 20’ for bored, 50 – 100’ deep for drilled, depending on class of well

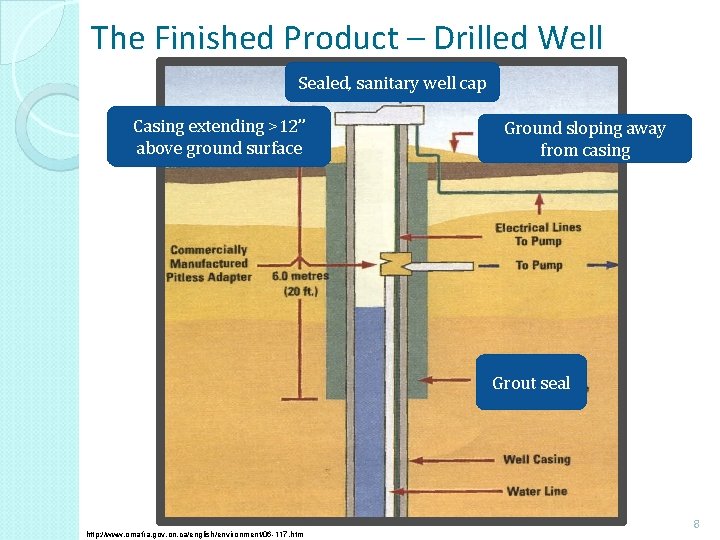
The Finished Product – Drilled Well Sealed, sanitary well cap Casing extending >12” above ground surface Ground sloping away from casing Grout seal http: //www. omafra. gov. on. ca/english/environment/06 -117. htm 8

Well Maintenance Tips Do not use fertilizers, pesticides, oil, or paint near well Keep area around well clean and accessible Keep careful records ◦ original contract, water test results and any maintenance or repair information Every year: ◦ Conduct thorough visual inspection of well ◦ Check cap for cracks, wear and tear, tightness Every 1 -3 years have well inspected by a qualified professional (with WWSP classification) 9

Private Water Supply Regulations • Virginia Private Well Regulations o Specify application, inspection and construction requirements o No requirements for maintenance or water testing after construction of well – responsibility of the owner! • EPA National Drinking Water Standards o Apply to PUBLIC systems o Primary (health) and Secondary (nuisance) o Can be used as guidance for private systems to know “how much is too much” 10

EPA Drinking Water Standards Primary Standards Secondary Standards • Also called Maximum Contaminant Level (MCL) Also called SMCL or RMCL Cause aesthetic problems: • Cause health problems o Staining • Enforced for public systems o Taste • Over 80 contaminants o Odor • For example: Can naturally occur in ground water About 15, including: o Nitrate o Lead o Coliform o Iron o Most organic chemicals and pesticides o Sulfate o Manganese 11

Testing water quality Why test? ◦ Protect family’s health and safety ◦ Many contaminants undetectable by human senses ◦ Preventive measures often more effective and less expensive ◦ Legal protection When to test? ◦ Routine tests every 1 -3 years ◦ Pregnant woman or infant in the home ◦ Recurring gastrointestinal illness ◦ Change in taste, appearance, odor of water ◦ Any services or repairs are done 12

What should I test for? Every year test for coliform bacteria ◦ Simple, relatively inexpensive test ◦ Indicates possible contamination from human or animal waste Every three years test: ◦ p. H (secondary std: 6. 5 – 8. 5) ◦ Total Dissolved Solids (TDS; secondary std 500 mg/L) ◦ Other contaminants based on local land uses nearby and condition of water 13

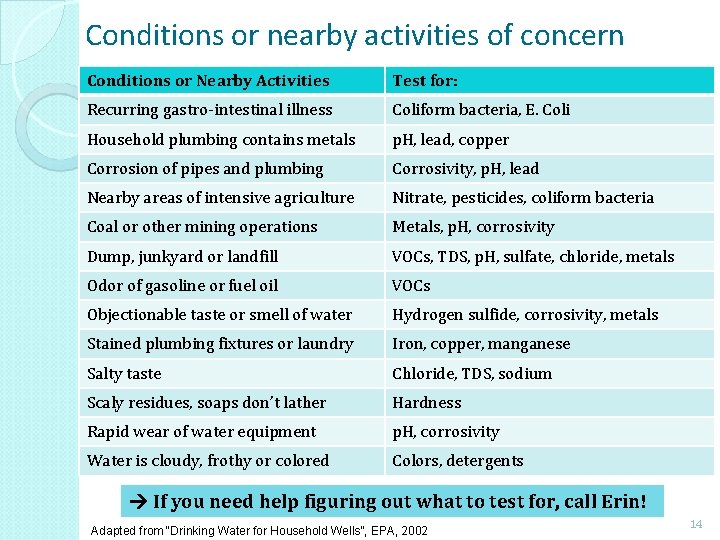
Conditions or nearby activities of concern Conditions or Nearby Activities Test for: Recurring gastro-intestinal illness Coliform bacteria, E. Coli Household plumbing contains metals p. H, lead, copper Corrosion of pipes and plumbing Corrosivity, p. H, lead Nearby areas of intensive agriculture Nitrate, pesticides, coliform bacteria Coal or other mining operations Metals, p. H, corrosivity Dump, junkyard or landfill VOCs, TDS, p. H, sulfate, chloride, metals Odor of gasoline or fuel oil VOCs Objectionable taste or smell of water Hydrogen sulfide, corrosivity, metals Stained plumbing fixtures or laundry Iron, copper, manganese Salty taste Chloride, TDS, sodium Scaly residues, soaps don’t lather Hardness Rapid wear of water equipment p. H, corrosivity Water is cloudy, frothy or colored Colors, detergents If you need help figuring out what to test for, call Erin! Adapted from “Drinking Water for Household Wells”, EPA, 2002 14

Understanding test results Most results provided as concentrations: ◦ mg/L (milligrams per liter) ≈ ppm (parts per million) ◦ µg/L = (micrograms per liter) ≈ ppb (parts per billion) Other units unique to test ◦ Radon, hardness, p. H Compare to EPA standards: http: //www. epa. gov/safewater/contaminants/index. html How much? 4 drops of ink in a 55 gallon barrel of water results in an “ink concentration” of 1 mg/L or ppm! 15

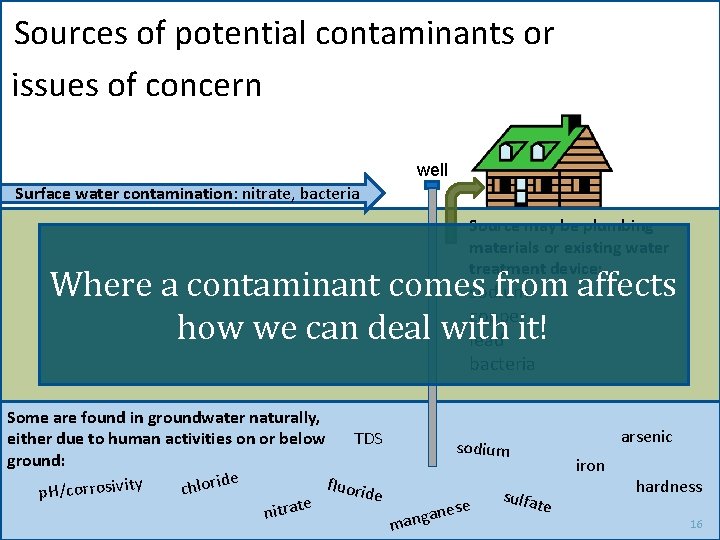
Sources of potential contaminants or issues of concern well Surface water contamination: nitrate, bacteria Source may be plumbing materials or existing water treatment device: Where a contaminant comessodium from affects copper how we can deal with lead it! bacteria Some are found in groundwater naturally, TDS either due to human activities on or below ground: ide r fluorid y it o l iv s o h r r o c c p. H/ e e t a r t ni arsenic sodium ese n a g man sulfat iron e hardness 16

Options for problem water 1. If possible, control the source of pollution ◦ Divert runoff, maintain septic system 2. Improve maintenance of water system ◦ Install sanitary well cap, slope the ground 3. Treat the water to reduce contaminant concentration ◦ Match the treatment option to the pollutant ◦ Consult a professional 4. Develop a new source of water ◦ Deeper well, develop spring, connect to water public 17 http: //static. howstuffworks. com/gif/septic-tank-cleaning-1. jpg, http: //www. shipewelldrilling. com/Pictures/well_drilling_rig. jpg, http: //www. clearflow. ca/REVERSE_OSMOSIS 2. jpg

Treatment Considerations Be sure to explore ALL of your options Always have water tested by a certified lab Be aware of unscrupulous businesses – look for National Sanitation Foundation (NSF) and Water Quality Association (WQA) certifications, consult Better Business Bureau (BBB) Point of Use (POU) vs. Point of Entry (POE) Weigh benefits and limitations of a device: ◦ Cost ◦ Maintenance requirements ◦ Warranty 18

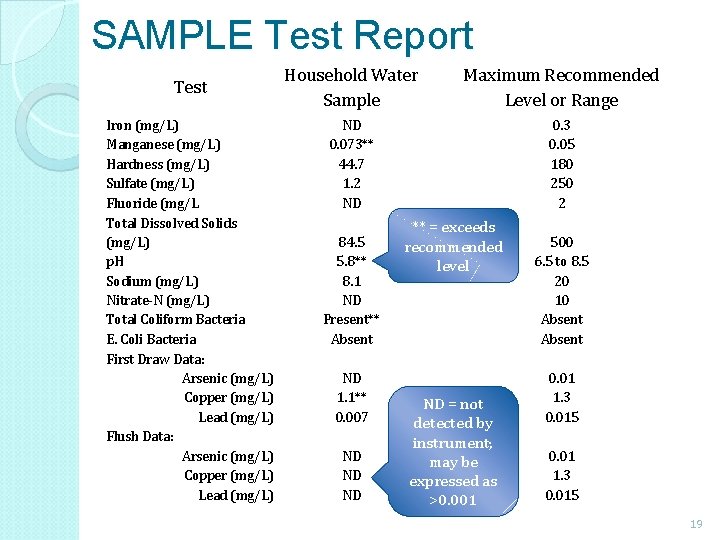
SAMPLE Test Report Test Iron (mg/L) Manganese (mg/L) Hardness (mg/L) Sulfate (mg/L) Fluoride (mg/L Total Dissolved Solids (mg/L) p. H Sodium (mg/L) Nitrate-N (mg/L) Total Coliform Bacteria E. Coli Bacteria First Draw Data: Arsenic (mg/L) Copper (mg/L) Lead (mg/L) Flush Data: Arsenic (mg/L) Copper (mg/L) Lead (mg/L) Household Water Sample Maximum Recommended Level or Range ND 0. 073** 44. 7 1. 2 ND 0. 3 0. 05 180 250 2 84. 5 5. 8** 8. 1 ND Present** Absent ND 1. 1** 0. 007 ND ND ND ** = exceeds recommended level ND = not detected by instrument; may be expressed as >0. 001 500 6. 5 to 8. 5 20 10 Absent 0. 01 1. 3 0. 015 19

Photo credits: www. water-research. net, www. britannica. com Coliform Bacteria Cannot be smelled, tasted or seen Coliform bacteria is an indicator organism – means disease-causing bacteria may be present Public standard is 0 colony forming units (cfu)/100 m. L (ABSENT) If present, test for fecal coliform or E. coli presence – indicator that sewage or animal waste is present. 20

If Coliform Bacteria are PRESENT Don’t panic! Examine well for pathways surface water can enter well (cracks in casing), make sure sanitary well cap is installed and secure, ground slopes away from well, etc. Consider shock chlorination Retest after shock chlorination Long term treatment options: ozonation, UV light, continuous chlorination 21

http: //www. kimicontrol. com/microorg/escherichia_coli. jpg If E. Coli Bacteria are PRESENT Take immediate steps to address Shock chlorinate Retest water In the meantime, consider boiling or use another source of water for drinking or cooking Check for potential contamination sources Consider long-term treatment options: UV light, ozonation, continuous disinfection 22

p. H Measure of the acidity or alkalinity of a substance (0 – 14) scale Logarithmic scale: p. H = 5 is 100 times more acidic than p. H = 7 Good indicator of general water quality Increasing acidity 0 1 2 Battery acid Gastric acid Lemon juice 3 Vinegar 4 Neutral 5 Coffee 6 Milk Distilled water 7 8 9 10 Increasing alkalinity 11 12 13 14 Recommended p. H range 6. 5 – 8. 5 Baking soda Sea water Milk of magnesia Ammonia Bleach Lye 23

Corrosive Water Also called aggressive water Corrodes metal plumbing – can leach metals, causes pitting and leaks, reduces length of appliance life Most commonly caused by low p. H; other contributing factors include alkalinity, temperature, TDS levels EPA recommends drinking water be non-corrosive Excess copper or lead in drinking water is a health concern Depending on p. H, treat with acid neutralizing filter or soda ash injection http: //www. bushman. cc/photos/Copper_Water_Pipe_Corrosion. jpg; http: //www. cee. vt. edu/ewr/environmental/teach/wtprimer/corrosion. html 24

Corrosive Water: Metals of concern Lead ◦ Many serious health effects, especially in children and infants Developmental, neurological, reproductive and renal ◦ EPA MCL is 0 mg/L with a health action level of 0. 015 mg/L. ◦ Sources include: Pipes in older homes (pre-1930) Solder in homes built prior to 1986 “Lead-free” brass fixtures (<8%) – even in NEW homes! Copper ◦ High levels can cause nausea, vomiting, stomach cramps; infants and children particularly sensitive ◦ EPA MCL is 1. 3 mg/L ◦ Nuisance effects noticeable at 1. 0 mg/L http: //www. gravitaexim. com/images/Lead-pipe. jpg 25

Addressing Lead or Copper in Water Options to consider: ◦ Discuss test results with your physician if concerned! ◦ Metals will be highest with corrosive water and contact time with pipes. Flushing pipes may address problem. Make sure that water runs until it is as cold as it gets before drinking ◦ Activated carbon filter (e. g. Brita) MAKE SURE IT IS LABELED TO REMOVE LEAD MAKE SURE TO CHANGE AS DIRECTED ◦ Address corrosivity of water – if p. H < 6. 5, can use acid neutralizing filter; however, corrosivity can be caused by other factors as well ◦ Reverse Osmosis ◦ Use another source of water known to be safe 26

http: //www. freedrinkingwater. com/images-water-quality/chemicals/water%20 in%20 reddish-brown. jpg Iron and Manganese Nuisance - not health concern SMCL: Iron = 0. 3 mg/L; Mn = 0. 05 mg/L Red-brown/black staining, particles, metallic taste Treatment depends on type/form of iron ◦ Ferrous: water initially clear orange-brown black solid particles ◦ Ferric: solid particles apparent immediately, water has a tint or or ◦ Iron bacteria: not a health concern; feed on Fe and Mn, forming red-brown or black-brown slime Treatment: water softener, aeration and filtration, ozonation, distillation 27

Fluoride Occurs naturally in varying levels ◦ Naturally high levels of F in E. Virginia groundwater Added to many public water systems for reduced dental caries and strong teeth and bones Health concerns: ◦ Long term exposure: links to bone cancer ◦ Shorter term exposure: dental or skeletal fluorosis EPA MCL 4. 0 mg/L and SMCL 2. 0 mg/L Optimum levels for public systems 0. 8 - 1. 2 mg/L Limited use for children up to 8 years Treatment (reverse osmosis) removes ALL fluoride http: //www. willamettedental. com/en_us/ALL/patients/pps/retailproducts_prettysmile. gif; http: //en. wikipedia. org/wiki/Dental_fluorosis 28

/www. cotrip. org/winterdriving/images/pic 6. jpg; /www. apswater. com/images/fleck%205600. jpg Sodium Low levels occur naturally; high levels may from man-made source be ◦ Road salt storage or application ◦ Industrial waste ◦ Sewage, fertilizers or animal waste ◦ WATER SOFTENER Sodium: EPA recommendation for people on low-sodium diets: 20 mg/L Consider other sources of salt in diet and discuss with Dr. Higher levels may indicate contamination – test for bacteria or other contaminants Salty taste; and may accelerate corrosion of pipes and water heaters Treat using distillation, reverse osmosis, demineralization 29

www. goodcleanwater. com/fyi. htm; www. watersoftening. org/effects_of_hard_water. htm; Hardness/Scaling Hard water contains high levels of and magnesium ions calcium ◦ Dissolved into water during contact with limestone and other minerals Not a health risk – nuisance ◦ Decreased cleaning action of soaps, detergents ◦ Scale build-up in pipes and on appliances ◦ Reduced efficiency and lifespan of water heaters No EPA standard for public systems Treat using water softener Hardness Rating Grains per Gallon mg/L Soft Less than 1. 0 Less than 17. 1 Slightly-Moderately Hard 1. 0 -7. 0 17. 1 -120 Hard 7. 0 -10. 5 120 -180 Very Hard Over 10. 5 Over 180 30

http: //wi. water. usgs. gov/pubs/FS-221 -95/p 2. gif Nitrate (NO 3 -N) Serious health concern for infants ◦ Methemoglobinemia or “blue baby syndrome” Nitrate nitrite during digestion and blood cannot carry oxygen ◦ MCL 10 mg/L NO 3 -N or 45 mg/L of NO 3 If 3 -5 mg/L, use do not use water for infants under 6 months Sources include fertilizer, animal manure, sewage NO 3 dissolves and moves easily through soil Test in spring months; levels change over time BOILING INCREASES concentration of nitrates!!! Treatment: distillation, reverse osmosis, ion exchange 31

Total Dissolved Solids (TDS) Water is a great solvent – dissolves many compounds as it travels over and under ground TDS is a measure of all dissolved impurities Natural sources: limestone, salt deposits, other minerals Man-made sources: ◦ Septic systems and sewage ◦ Run off from agricultural or urban land ◦ Road salt, industrial sources General indicator of water quality; test at least every three years EPA SMCL is 500 mg/L Treat using distillation or reverse osmosis http: //en. wikipedia. org/wiki/Total_dissolved_solids 32

Arsenic Occurs naturally in some rocks; more common in groundwater supplies when water tables rise and fall frequently Used in wood preservatives, paints, pesticides, etc. Linked to many types of cancer, stomach pain, paralysis, and blindness EPA primary standard is 0. 010 mg/L Reverse osmosis to remove 33

Virginia Master Well Owner Network Training Workshop ore m n our r a Le out y ! ab ater w Want to learn more about your private water supply? Help Other s! Visit www. wellwater. bse. vt. edu Contact Erin Ling wellwater@vt. edu Free rce u o s Re er! Bind 540 -231 -9058 y Appl ! today 34

Virginia Household Water Quality Program Virginia Master Well Owner Network Erin Ling (wellwater@vt. edu) Brian Benham (benham@vt. edu) www. wellwater. bse. vt. edu Email: wellwater@vt. edu Ph: 540 -231 -9058 35

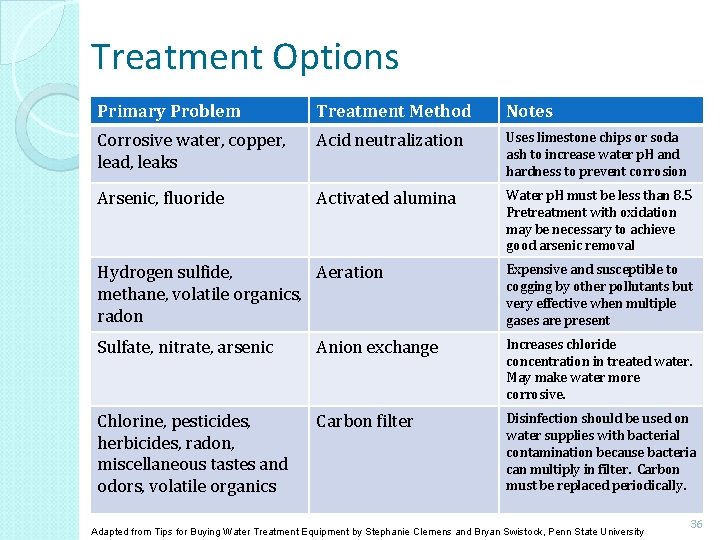
Treatment Options Primary Problem Treatment Method Notes Corrosive water, copper, lead, leaks Acid neutralization Uses limestone chips or soda ash to increase water p. H and hardness to prevent corrosion Arsenic, fluoride Activated alumina Water p. H must be less than 8. 5 Pretreatment with oxidation may be necessary to achieve good arsenic removal Hydrogen sulfide, Aeration methane, volatile organics, radon Expensive and susceptible to cogging by other pollutants but very effective when multiple gases are present Sulfate, nitrate, arsenic Anion exchange Increases chloride concentration in treated water. May make water more corrosive. Chlorine, pesticides, herbicides, radon, miscellaneous tastes and odors, volatile organics Carbon filter Disinfection should be used on water supplies with bacterial contamination because bacteria can multiply in filter. Carbon must be replaced periodically. Adapted from Tips for Buying Water Treatment Equipment by Stephanie Clemens and Bryan Swistock, Penn State University 36

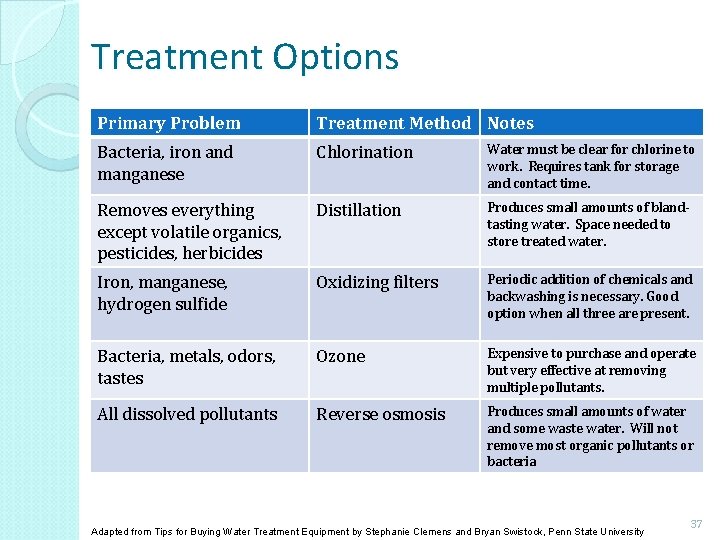
Treatment Options Primary Problem Treatment Method Notes Bacteria, iron and manganese Chlorination Water must be clear for chlorine to work. Requires tank for storage and contact time. Removes everything except volatile organics, pesticides, herbicides Distillation Produces small amounts of blandtasting water. Space needed to store treated water. Iron, manganese, hydrogen sulfide Oxidizing filters Periodic addition of chemicals and backwashing is necessary. Good option when all three are present. Bacteria, metals, odors, tastes Ozone Expensive to purchase and operate but very effective at removing multiple pollutants. All dissolved pollutants Reverse osmosis Produces small amounts of water and some waste water. Will not remove most organic pollutants or bacteria Adapted from Tips for Buying Water Treatment Equipment by Stephanie Clemens and Bryan Swistock, Penn State University 37

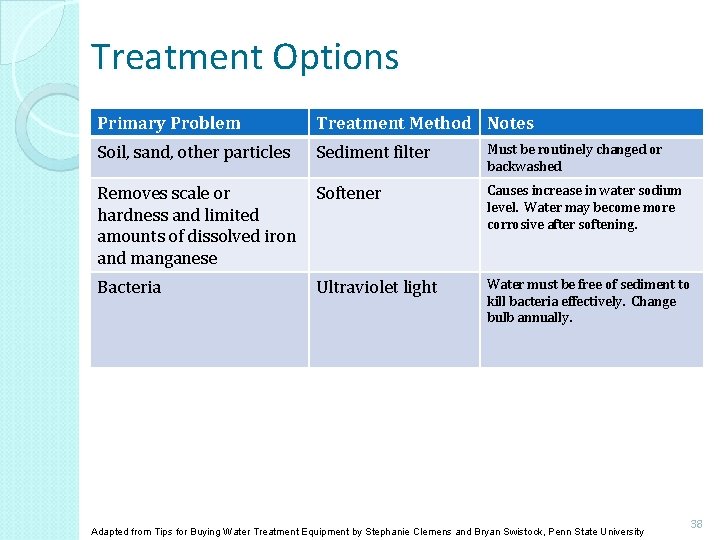
Treatment Options Primary Problem Treatment Method Notes Soil, sand, other particles Sediment filter Must be routinely changed or backwashed Removes scale or hardness and limited amounts of dissolved iron and manganese Softener Causes increase in water sodium level. Water may become more corrosive after softening. Bacteria Ultraviolet light Water must be free of sediment to kill bacteria effectively. Change bulb annually. Adapted from Tips for Buying Water Treatment Equipment by Stephanie Clemens and Bryan Swistock, Penn State University 38
 Erin ling
Erin ling Water and water and water water
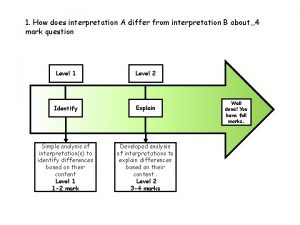
Water and water and water water How does interpretation b differ from interpretation a
How does interpretation b differ from interpretation a Tceq water watch
Tceq water watch Drinking water state revolving fund
Drinking water state revolving fund Oregon wastewater operator certification
Oregon wastewater operator certification Idem drinking water watch
Idem drinking water watch Tceq water watch
Tceq water watch Lithium in drinking water
Lithium in drinking water Typhoid medicine course
Typhoid medicine course Drinking water system operator certificate
Drinking water system operator certificate Prime drinking water
Prime drinking water Nm drinking water watch
Nm drinking water watch What is meeting and types of meeting
What is meeting and types of meeting Types of meeting
Types of meeting Today meeting or today's meeting
Today meeting or today's meeting Today meeting or today's meeting
Today meeting or today's meeting Jin ling cigarettes
Jin ling cigarettes Qüvvədə qazanc
Qüvvədə qazanc Ling
Ling Ling oa
Ling oa Mei-ling from singapore was preparing
Mei-ling from singapore was preparing Hija de patty y selma
Hija de patty y selma Dr ng li ling
Dr ng li ling Nien-ling wacker
Nien-ling wacker Ling shih fu
Ling shih fu Ling simpson
Ling simpson Ling adder
Ling adder Father of physical education in denmark
Father of physical education in denmark Walter ling
Walter ling Ling
Ling Ling
Ling Ling
Ling Mtling
Mtling Wai ling lam
Wai ling lam Ling oa
Ling oa Wang ling relationship
Wang ling relationship Huo lingyu
Huo lingyu Ling rolled
Ling rolled