Drill Draw the Lewis Dot Diagram for C























- Slides: 37

Drill: Draw the Lewis Dot Diagram for C 6 H 8

Review Drill & Check HW

Organic HW • Review PP-2 • Complete the attached assignment & turn it in tomorrow.

Alkanes

Alkanes • Hydrocarbons containing only single covalent bonds

Alkanes • All hydrocarbons with no multiple bonds end with the suffix -ane • Prefix: Alk- any length carbon chain

Organic Prefixes • 1 = • 2 = • 3 = • 4 = • 5 = methethpropbutpent- 6= 7= 8= 9= 10 = hexheptoctnondec-

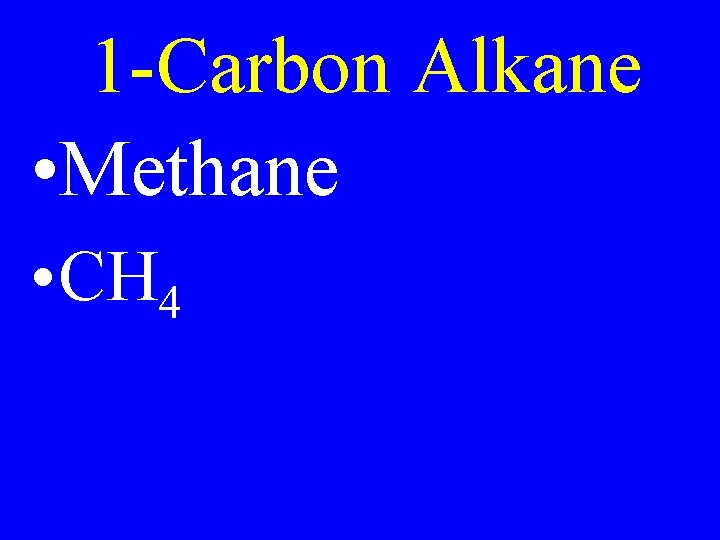
1 -Carbon Alkane • Methane • CH 4

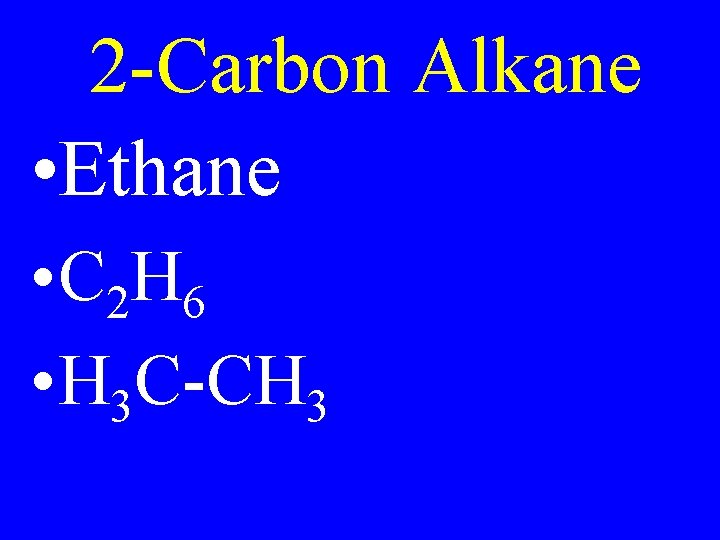
2 -Carbon Alkane • Ethane • C 2 H 6 • H 3 C-CH 3

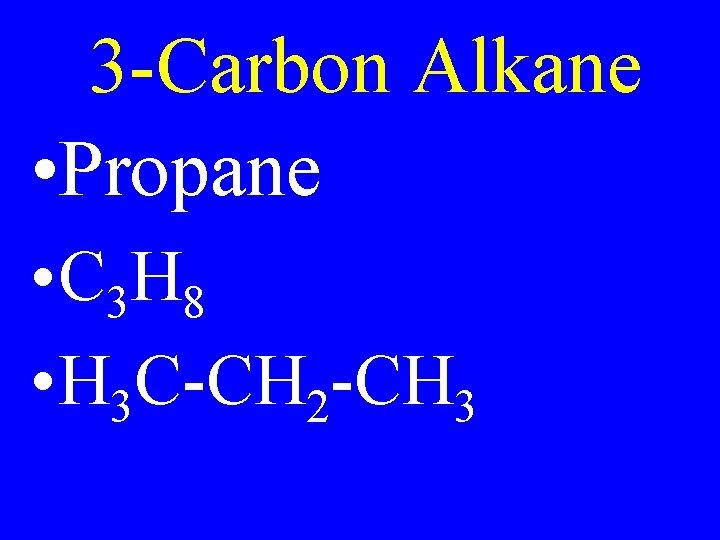
3 -Carbon Alkane • Propane • C 3 H 8 • H 3 C-CH 2 -CH 3

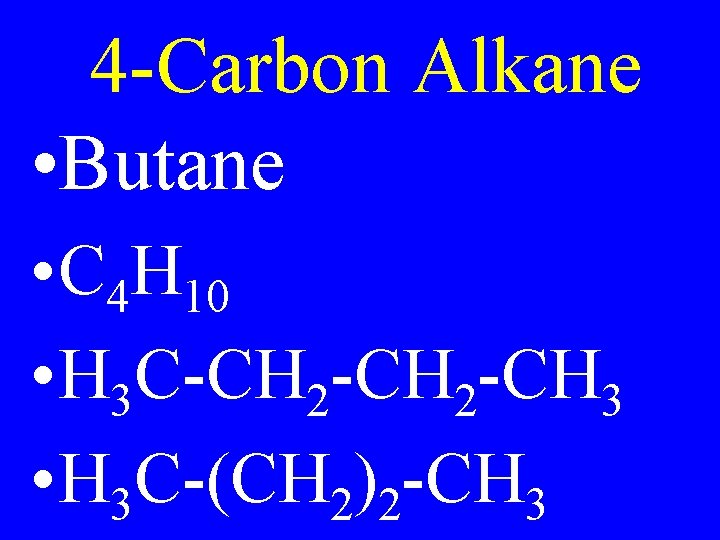
4 -Carbon Alkane • Butane • C 4 H 10 • H 3 C-CH 2 -CH 3 • H 3 C-(CH 2)2 -CH 3

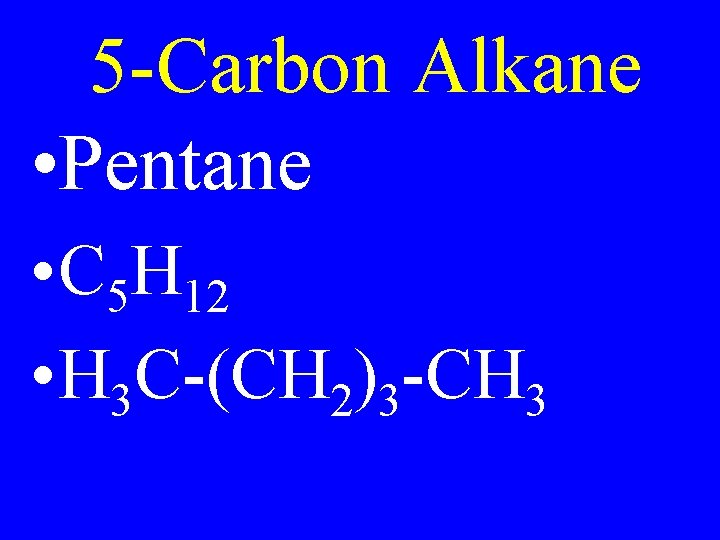
5 -Carbon Alkane • Pentane • C 5 H 12 • H 3 C-(CH 2)3 -CH 3

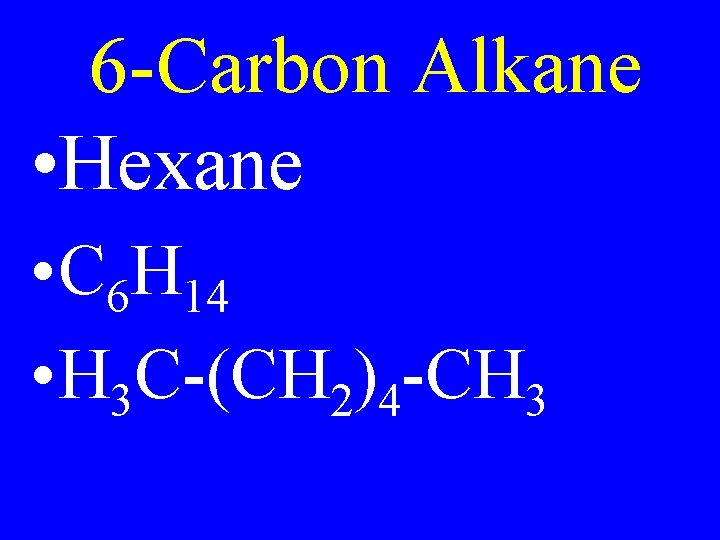
6 -Carbon Alkane • Hexane • C 6 H 14 • H 3 C-(CH 2)4 -CH 3

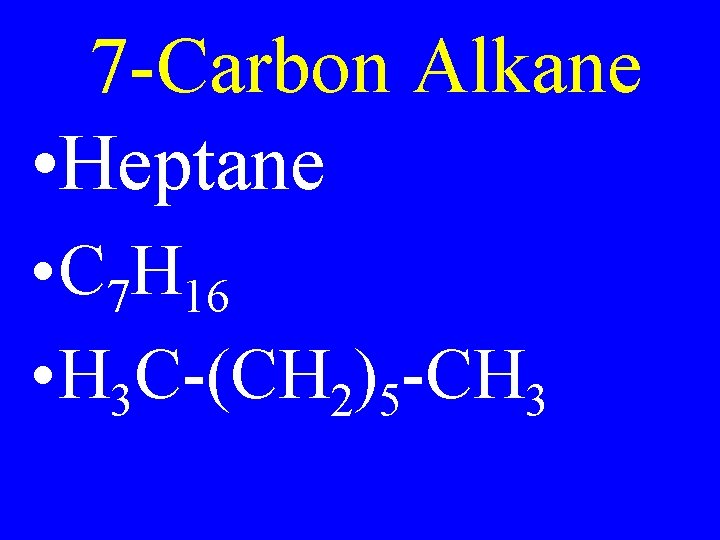
7 -Carbon Alkane • Heptane • C 7 H 16 • H 3 C-(CH 2)5 -CH 3

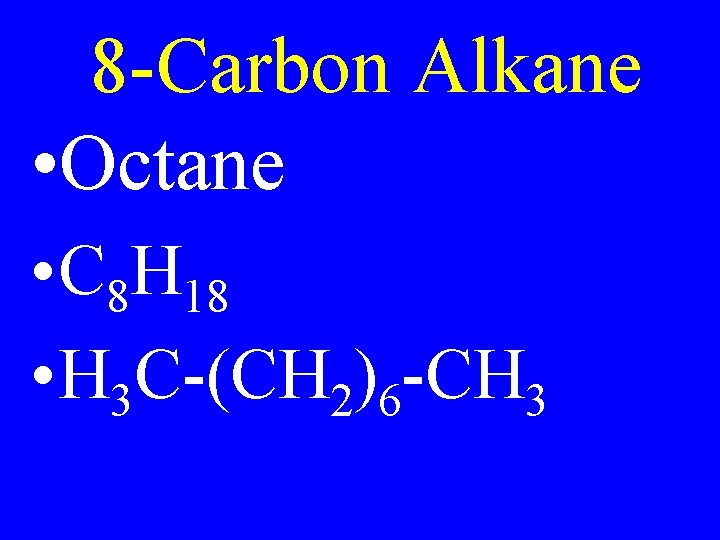
8 -Carbon Alkane • Octane • C 8 H 18 • H 3 C-(CH 2)6 -CH 3

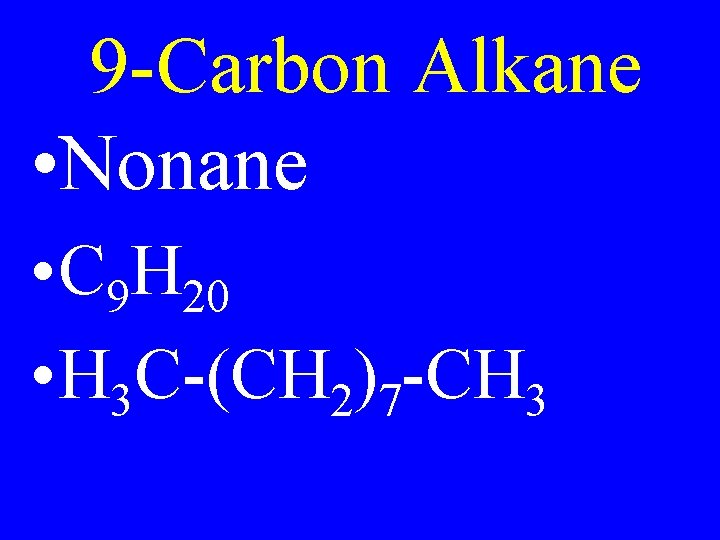
9 -Carbon Alkane • Nonane • C 9 H 20 • H 3 C-(CH 2)7 -CH 3

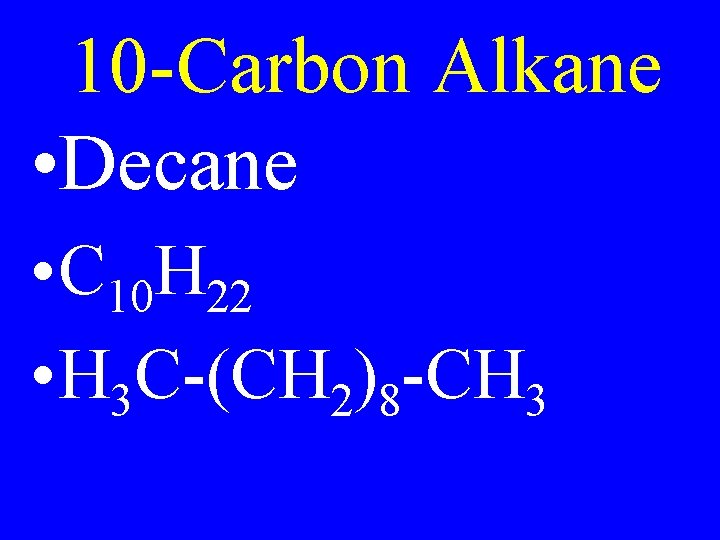
10 -Carbon Alkane • Decane • C 10 H 22 • H 3 C-(CH 2)8 -CH 3

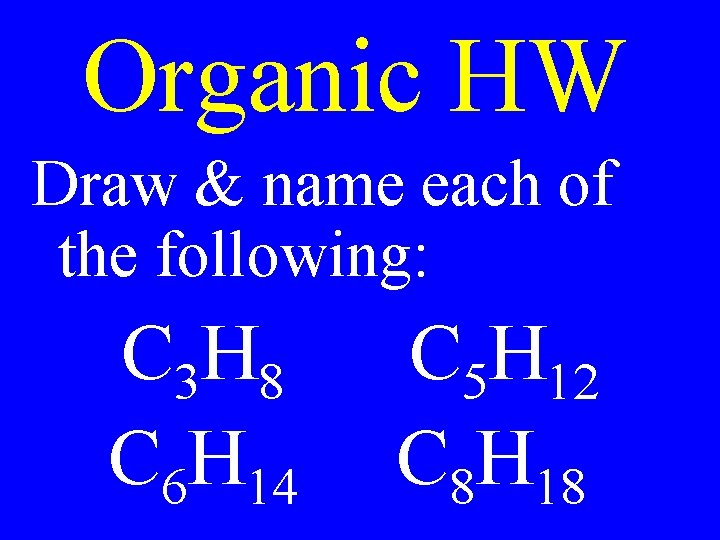
Organic HW Draw & name each of the following: C 3 H 8 C 6 H 14 C 5 H 12 C 8 H 18

Drill • Name & give the molecular formula for alkanes with 1, 2, 3, 4, & 5 carbons

Alkane Chemical Formula Cn. H 2 n+2

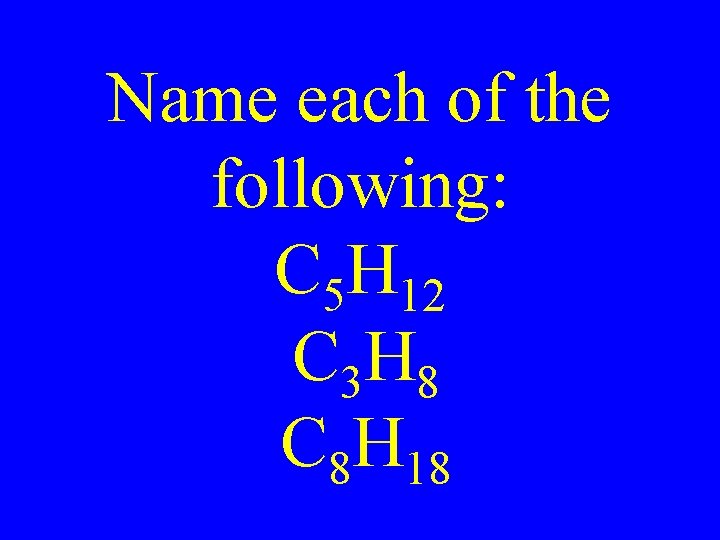
Name each of the following: C 5 H 12 C 3 H 8 C 8 H 18

Saturated Hydrocarbon • A hydrocarbon with the maximum number of hydrogens possible

All Pure Noncyclic Alkanes • Are saturated hydrocarbons

Carbon Chains • Any continuous chain hydrocarbon is called a carbon chain

Drill Draw & name 2 carbon chains. (1 even & 1 odd numbered > 2 C)

Formula Writing • Molecular Formula C 4 H 10

Formula Writing • Condensed formula: • C-C & C-H bonds understood CH 3 CH 2 CH 3

Formula Writing • Condensed formula: • only C-H bonds understood CH 3 -CH 2 -CH 3

Formula Writing • Condensed formula: • bonds understood & repeats in () CH 3(CH 2)2 CH 3

Formula Writing Skeletal Formula • Carbon skeleton, C-H bonds omitted C-C-C-C

Formula Writing • Complete structural formula HHHH H C C H HHHH

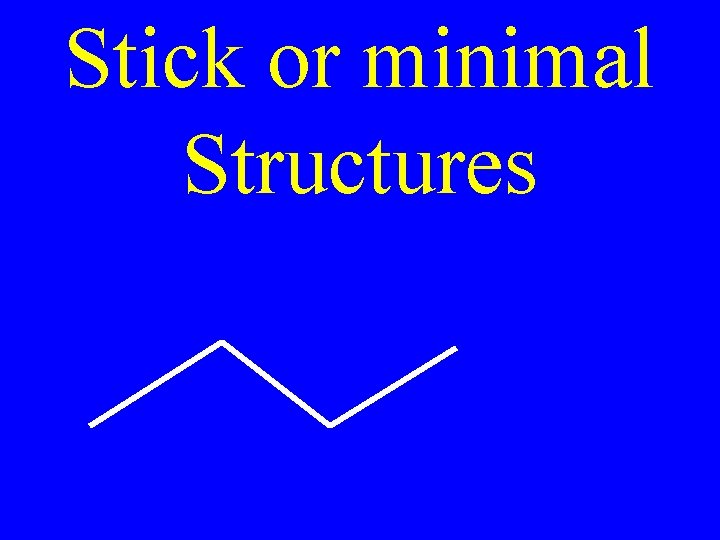
Stick or minimal Structures

Stick Structures • Line ends & joints represent carbons • Hydrogens understood • Others draw

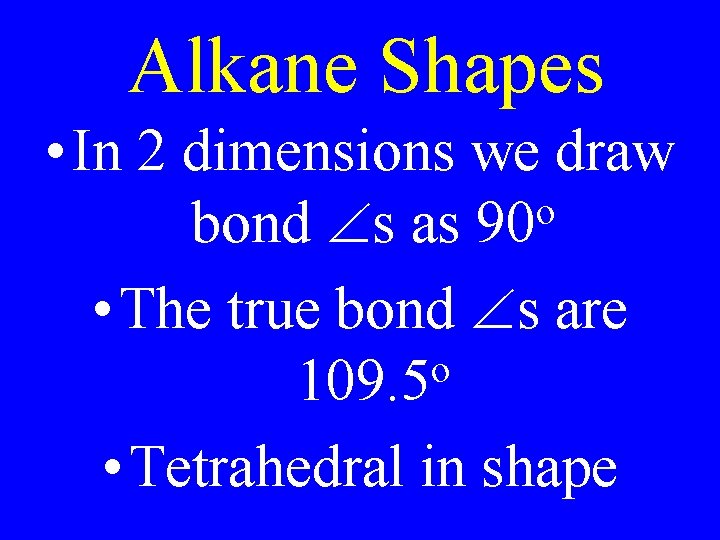
Alkane Shapes • In 2 dimensions we draw o bond s as 90 • The true bond s are o 109. 5 • Tetrahedral in shape

Organic HW • Draw & name 3 different alkanes, making sure that no two drawing are done by the same method.

Draw the following • Propane • hexane • butane • heptane octane pentane methane

Name the following