DRAWING OF AN ATOM ELECTRON ARRANGEMENT PG 25



- Slides: 17

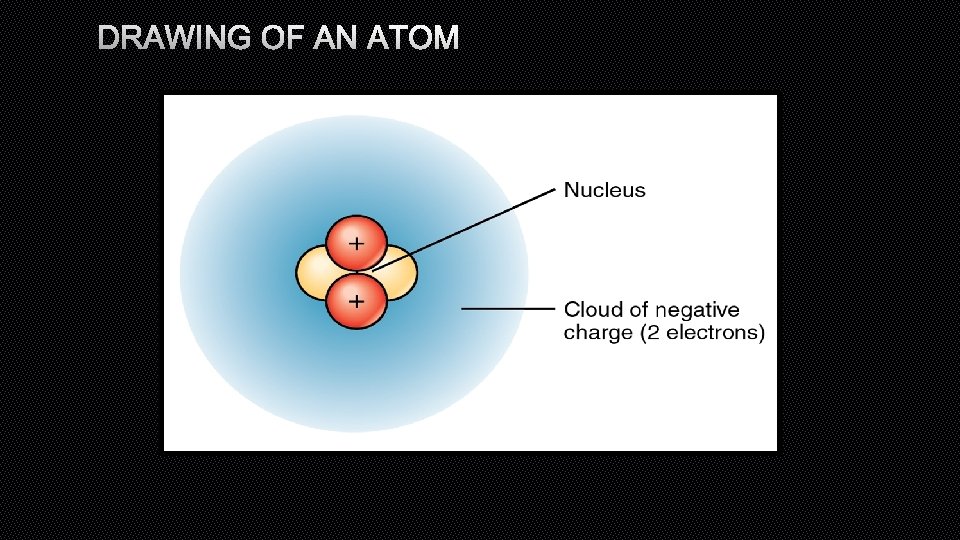
DRAWING OF AN ATOM

ELECTRON ARRANGEMENT PG. 25



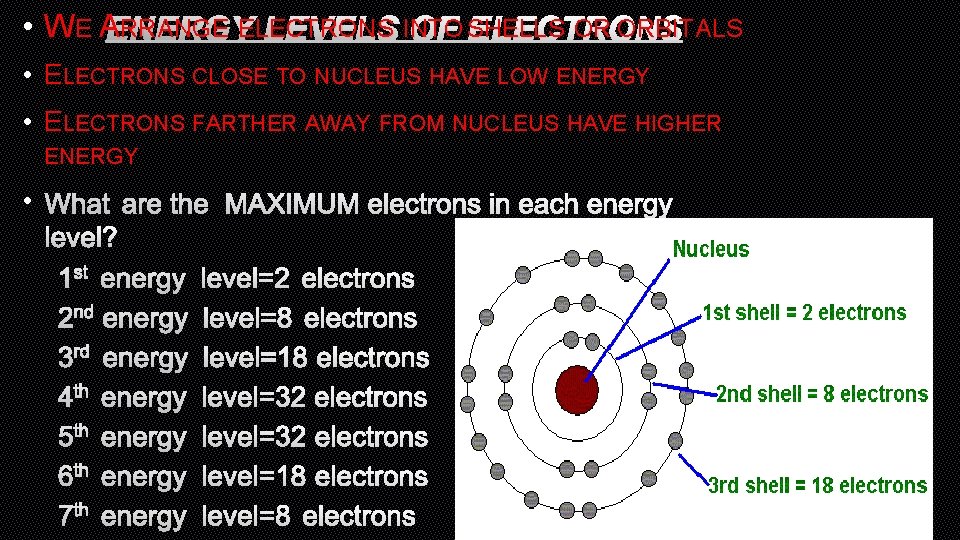
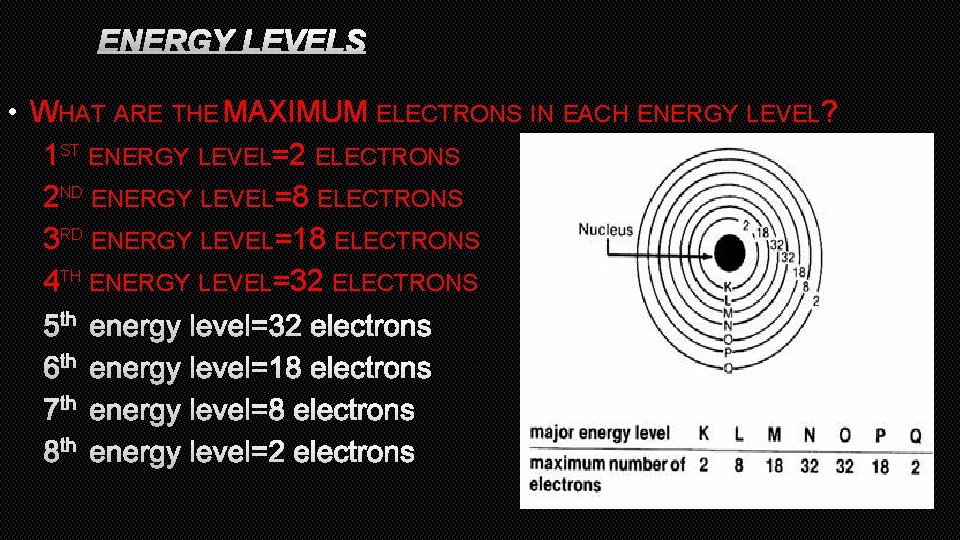
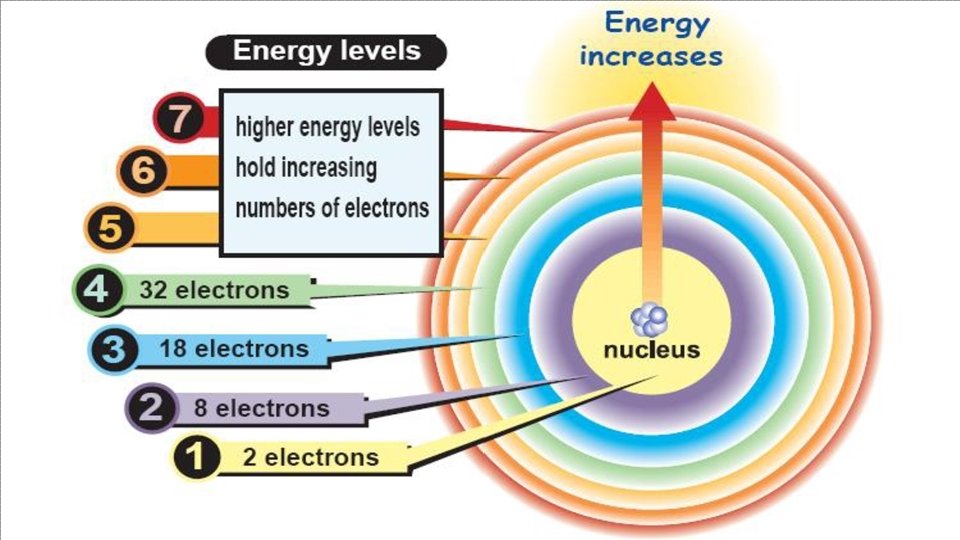
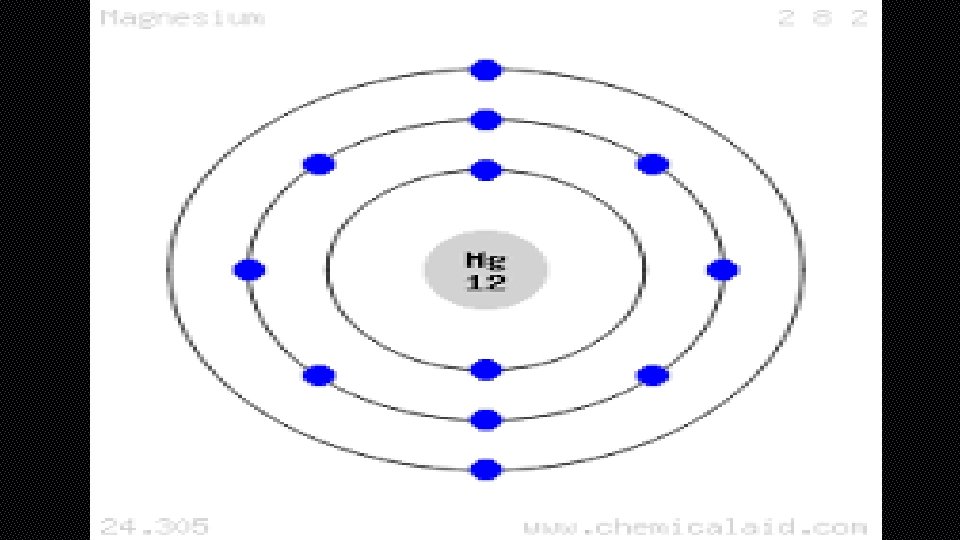
• WE AENERGY RRANGE ELECTRONS OR ORBITALS LEVELS INTO OF SHELLS ELECTRONS • ELECTRONS CLOSE TO NUCLEUS HAVE LOW ENERGY • ELECTRONS FARTHER AWAY FROM NUCLEUS HAVE HIGHER ENERGY • WHAT ARE THE MAXIMUM ELECTRONS IN EACH ENERGY LEVEL? 1 ST ENERGY LEVEL=2 ELECTRONS 2 ND ENERGY LEVEL=8 ELECTRONS 3 RD ENERGY LEVEL=18 ELECTRONS 4 TH ENERGY LEVEL=32 ELECTRONS 5 TH ENERGY LEVEL=32 ELECTRONS 6 TH ENERGY LEVEL=18 ELECTRONS 7 TH ENERGY LEVEL=8 ELECTRONS

ENERGY LEVELS • WHAT ARE THE MAXIMUM ELECTRONS IN EACH ENERGY LEVEL? 1 ST ENERGY LEVEL=2 ELECTRONS 2 ND ENERGY LEVEL=8 ELECTRONS 3 RD ENERGY LEVEL=18 ELECTRONS 4 TH ENERGY LEVEL=32 ELECTRONS 5 TH ENERGY LEVEL=32 ELECTRONS 6 TH ENERGY LEVEL=18 ELECTRONS 7 TH ENERGY LEVEL=8 ELECTRONS 8 TH ENERGY LEVEL=2 ELECTRONS


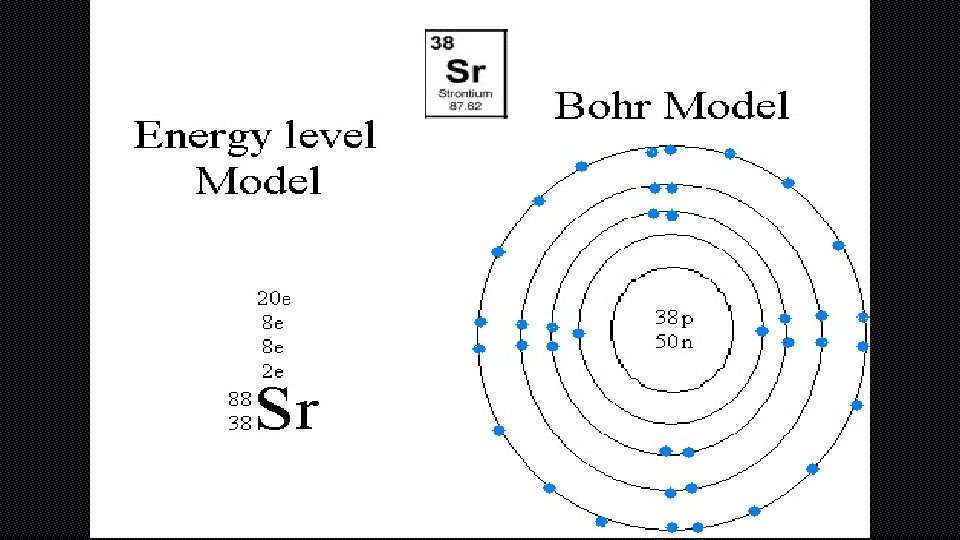
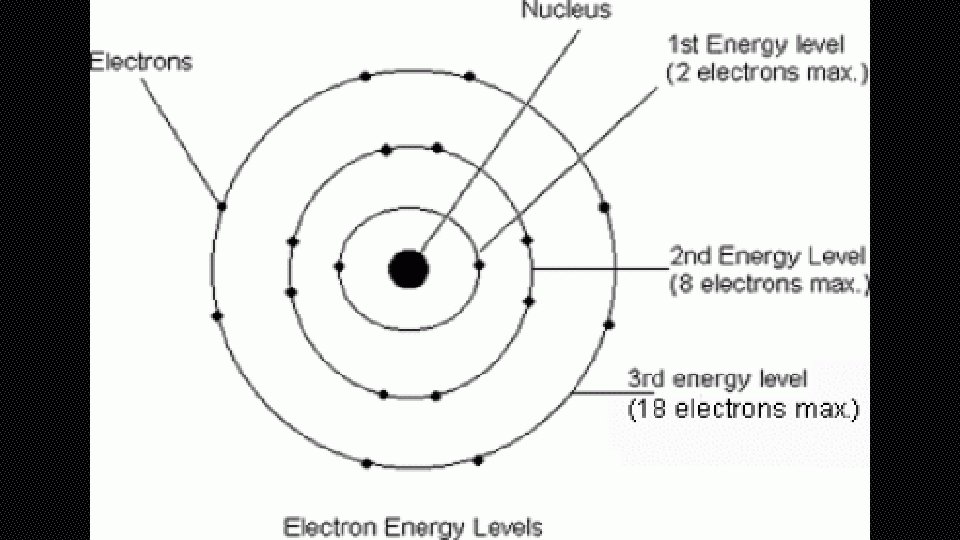
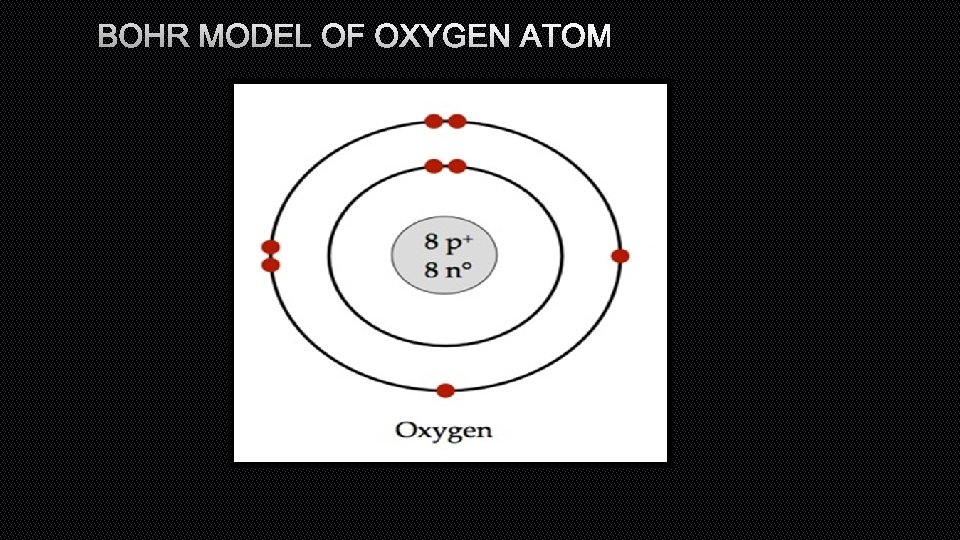
BOHR DIAGRAM • BOHR DIAGRAMS SHOW EACH OF THE ENERGY LEVELS OF THE ATOM • SHOW EVERY ELECTRON THAT THE ATOM HAS ORBITING AROUND IT • THESE ELECTRONS ARE ORGANIZED INTO THE DIFFERENT SHELLS OR ORBITAL LEVELS • 1 ST LEVEL=2 ELECTRONS • 2 ND LEVEL=8 ELECTRONS • 3 RD LEVEL=18 ELECTRONS





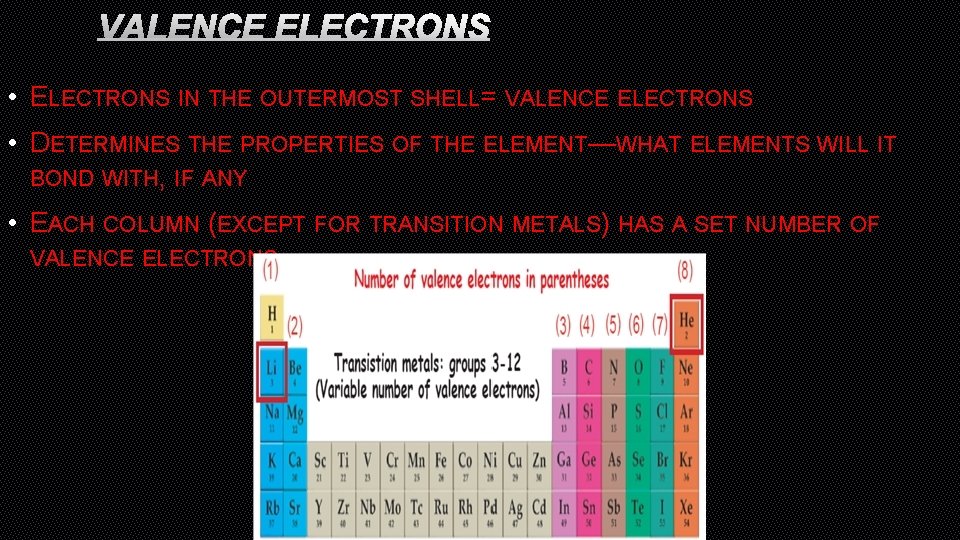
VALENCE ELECTRONS • ELECTRONS IN THE OUTERMOST SHELL= VALENCE ELECTRONS • DETERMINES THE PROPERTIES OF THE ELEMENT—WHAT ELEMENTS WILL IT BOND WITH, IF ANY • EACH COLUMN (EXCEPT FOR TRANSITION METALS) HAS A SET NUMBER OF VALENCE ELECTRONS

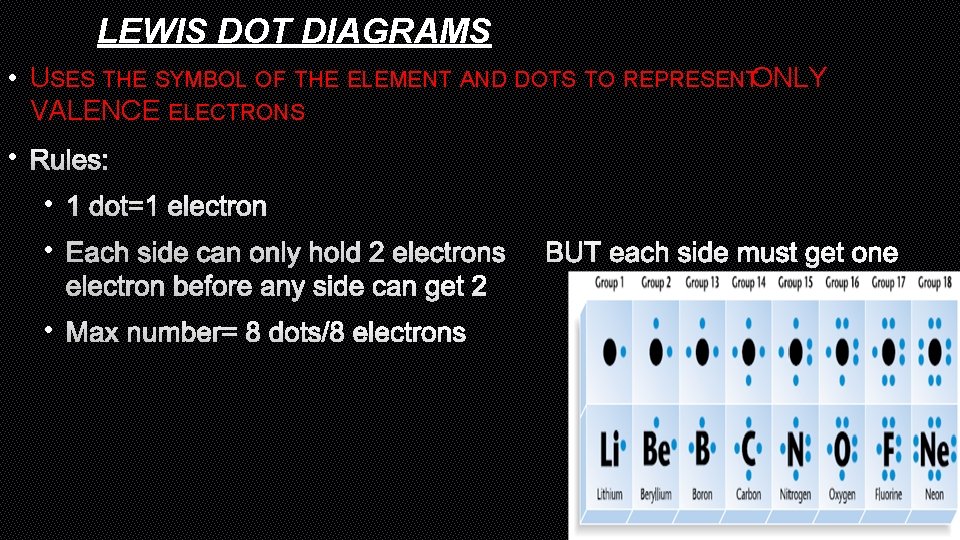
LEWIS DOT DIAGRAMS • USES THE SYMBOL OF THE ELEMENT AND DOTS TO REPRESENTONLY VALENCE ELECTRONS • RULES: • 1 DOT=1 ELECTRON • EACH SIDE CAN ONLY HOLD 2 ELECTRONSBUT EACH SIDE MUST GET ONE ELECTRON BEFORE ANY SIDE CAN GET 2 • MAX NUMBER= 8 DOTS/8 ELECTRONS

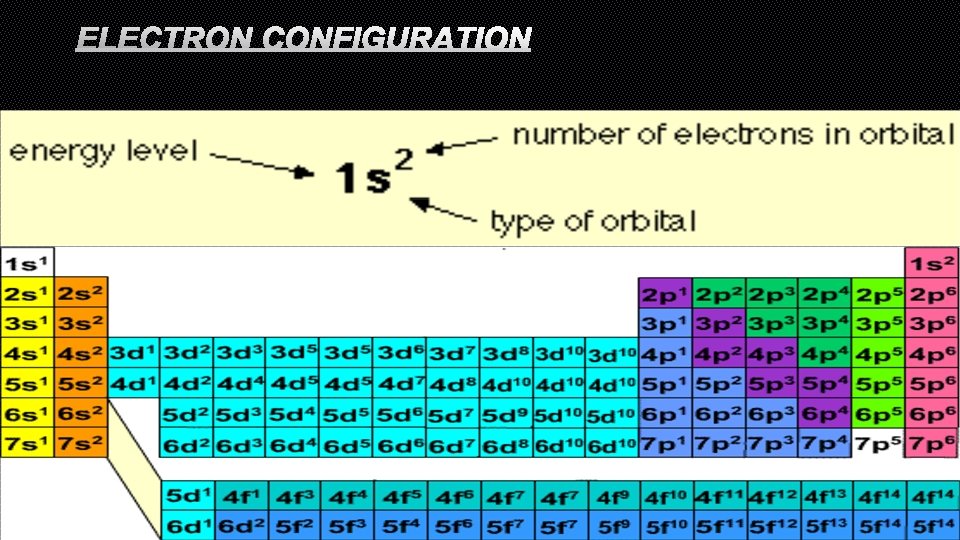
ELECTRON CONFIGURATION

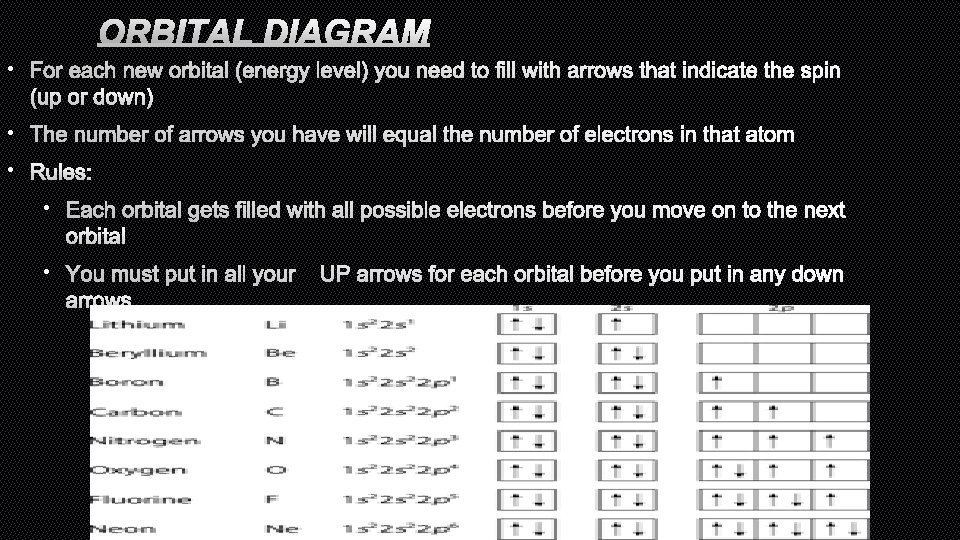
ORBITAL DIAGRAM • FOR EACH NEW ORBITAL (ENERGY LEVEL) YOU NEED TO FILL WITH ARROWS THAT INDICATE THE SPIN (UP OR DOWN) • THE NUMBER OF ARROWS YOU HAVE WILL EQUAL THE NUMBER OF ELECTRONS IN THAT ATOM • RULES: • EACH ORBITAL GETS FILLED WITH ALL POSSIBLE ELECTRONS BEFORE YOU MOVE ON TO THE NEXT ORBITAL • YOU MUST PUT IN ALL YOURUP ARROWS FOR EACH ORBITAL BEFORE YOU PUT IN ANY DOWN ARROWS

BOHR MODEL OF OXYGEN ATOM