Drawing Molecular Dot Diagrams Important Information Covalent compounds







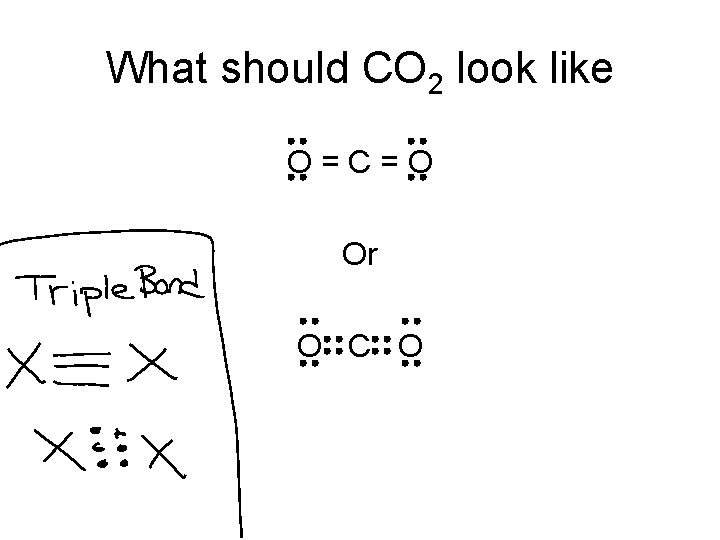

- Slides: 19

Drawing Molecular Dot Diagrams

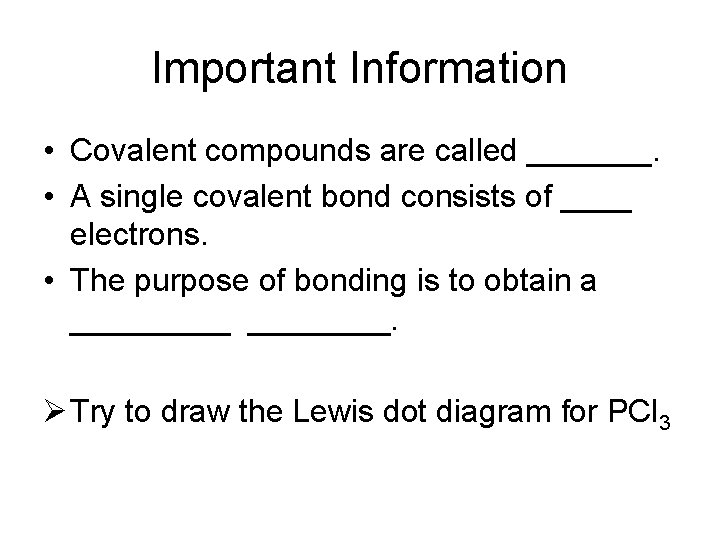
Important Information • Covalent compounds are called _______. • A single covalent bond consists of ____ electrons. • The purpose of bonding is to obtain a _____. Ø Try to draw the Lewis dot diagram for PCl 3

PCl 3 1. Arrange bonding atoms. • • If only 2 atoms, they go next to each other If there is a central atom, place it in the middle and outside atoms around it.

PCl 3 2. Count the total number of valence electrons. Write this number down.

PCl 3 3. Place a bond between each neighboring pair of atoms (single bond).

PCl 3 3 b. Replace each set of paired electrons with a line (This is not completely necessary).

PCl 3 4. Give all the atoms in the compound a stable octet (duet for hydrogen).

PCl 3 5. CHECK!!! A. Does everything have a stable octet (duet for hydrogen)? B. Do you have the correct number of valence electrons? Count the total number of dots (remember that each line represents 2 dots) and make sure that this number is the same as the one you counted in step #3.

Now try HCl

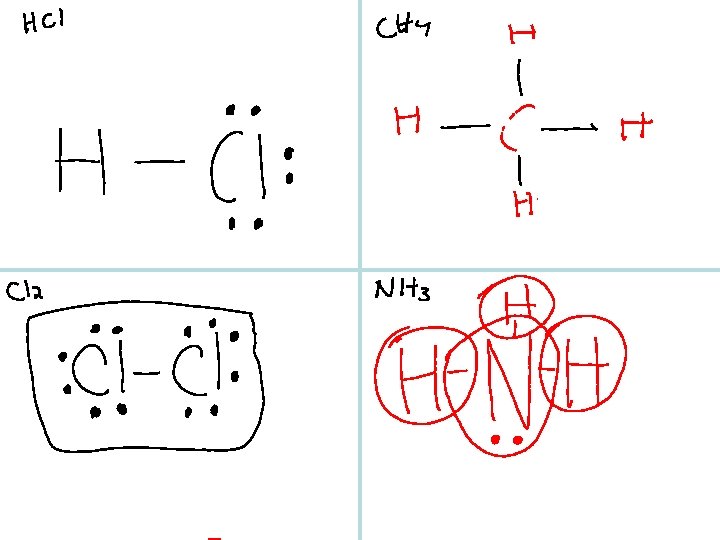
Before Thanksgiving we learned to draw Lewis dot diagrams for molecular compounds. 1. Look over your notes and the rules to draw these diagrams. 2. Make sure you understand the PCl 3 example from last Tuesday. 3. Try to draw – – HCl CH 4 Cl 2 NH 3

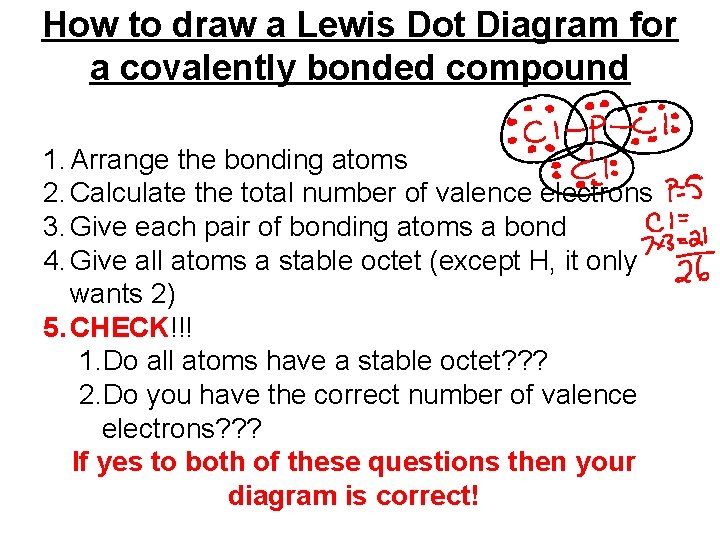
How to draw a Lewis Dot Diagram for a covalently bonded compound 1. Arrange the bonding atoms 2. Calculate the total number of valence electrons 3. Give each pair of bonding atoms a bond 4. Give all atoms a stable octet (except H, it only wants 2) 5. CHECK!!! 1. Do all atoms have a stable octet? ? ? 2. Do you have the correct number of valence electrons? ? ? If yes to both of these questions then your diagram is correct!


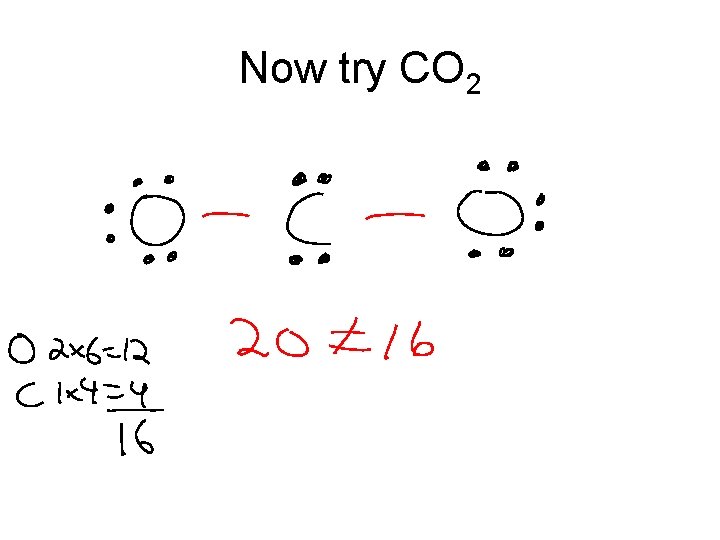
Now try CO 2

How to draw a Lewis Dot Diagram for a covalently bonded compound 1. Arrange the bonding atoms 2. Calculate the total number of valence electrons 3. Give each pair of bonding atoms a bond 4. Give all atoms a stable octet (except H, it only wants 2) 5. CHECK!!! 1. Do all atoms have a stable octet? ? ? 2. Do you have the correct number of valence electrons? ? ? If yes to both of these questions then your diagram is correct! If you have answered no…. . They you need a multiple covalent bond!

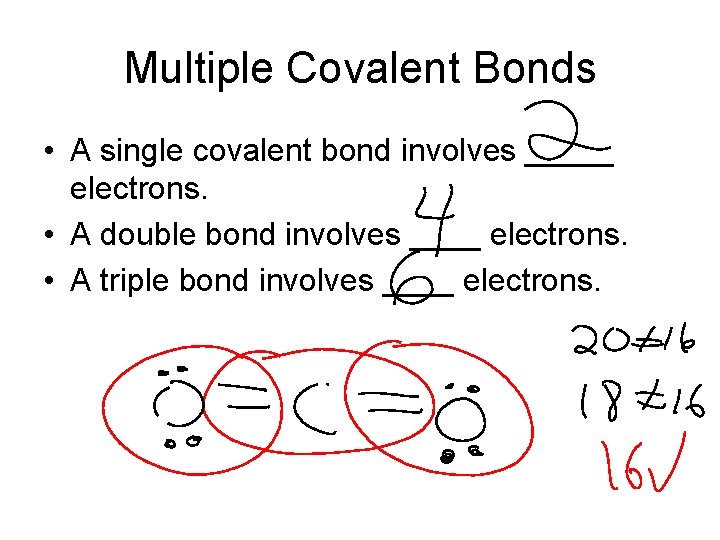
Multiple Covalent Bonds • A single covalent bond involves _____ electrons. • A double bond involves ____ electrons. • A triple bond involves ____ electrons.

CO 2 O–C-O • All atoms satisfy the octet rule but you have the wrong # of dots. • Remove 4 electrons (2 on each from bonded atoms and replace with an additional bond • Recheck! (Check octet rule and total # of electrons)
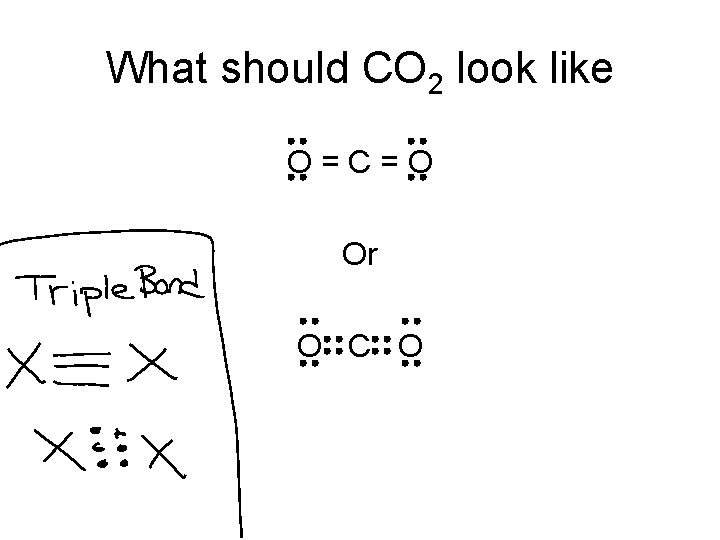
What should CO 2 look like O=C=O Or O C O

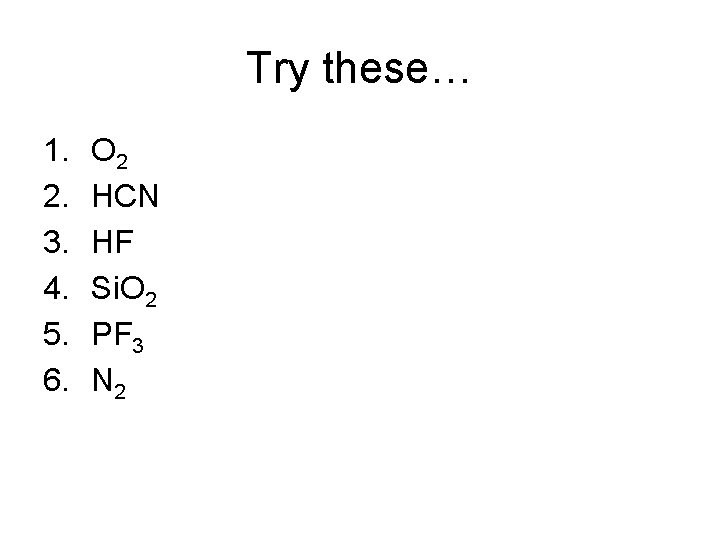
Try these… 1. 2. 3. 4. 5. 6. O 2 HCN HF Si. O 2 PF 3 N 2

Tomorrow we discuss the shapes of different molecules.