Drawing atoms using the ID card Homework is






- Slides: 12

Drawing atoms using the ID card. Homework is the worksheet.

Homework review

ID numbers today!! Each of you will get a number for looking at grade reports on the mean machine display. Write this number in two places in your notebook so you will have it if you forget it.

Today: Electron Configuration Drawing the Atom 1. Circle dot diagrams 2. Lewis dot diagrams Even though the atom is complex, we still use certain simple models to draw atoms. It is called a circle dot diagram. It has other names too.

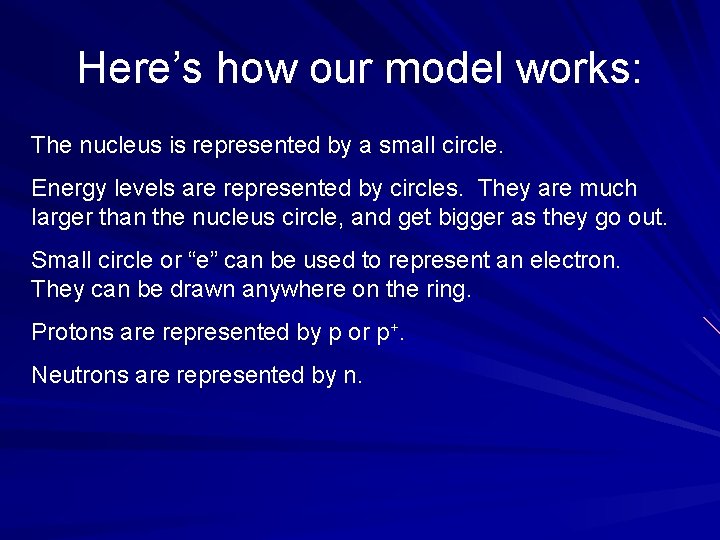
Here’s how our model works: The nucleus is represented by a small circle. Energy levels are represented by circles. They are much larger than the nucleus circle, and get bigger as they go out. Small circle or “e” can be used to represent an electron. They can be drawn anywhere on the ring. Protons are represented by p or p+. Neutrons are represented by n.

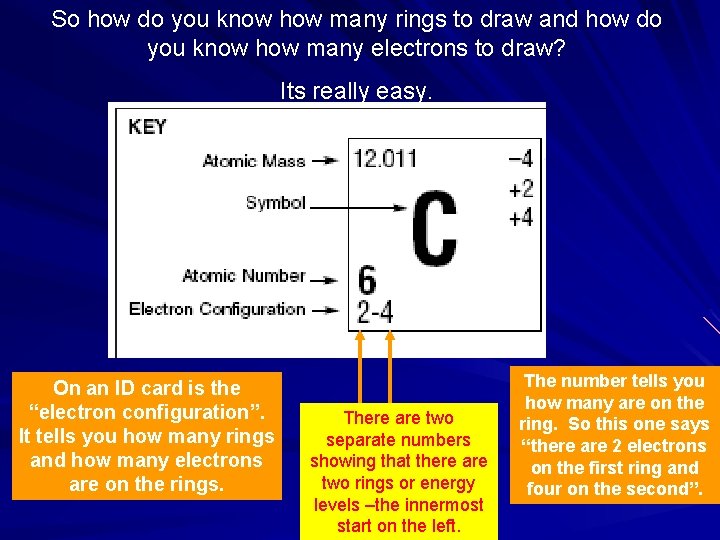
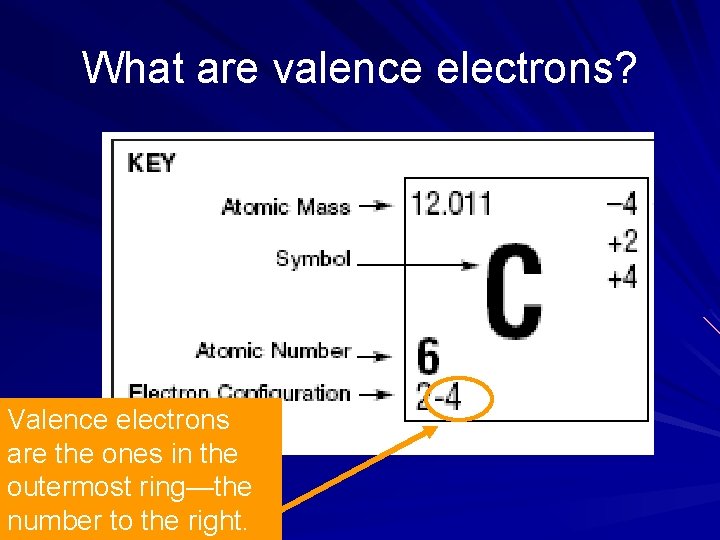
So how do you know how many rings to draw and how do you know how many electrons to draw? Its really easy. On an ID card is the “electron configuration”. It tells you how many rings and how many electrons are on the rings. There are two separate numbers showing that there are two rings or energy levels –the innermost start on the left. The number tells you how many are on the ring. So this one says “there are 2 electrons on the first ring and four on the second”.

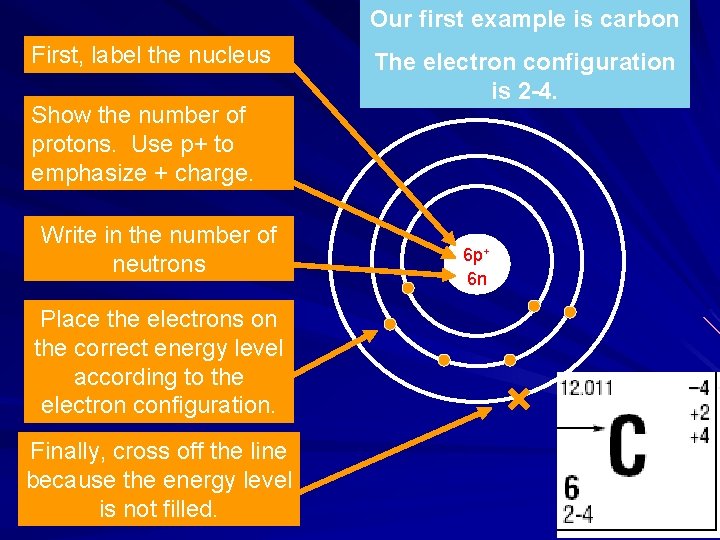
Our first example is carbon First, label the nucleus Show the number of protons. Use p+ to emphasize + charge. Write in the number of neutrons Place the electrons on the correct energy level according to the electron configuration. Finally, cross off the line because the energy level is not filled. The electron configuration is 2 -4. 6 p+ 6 n

Lewis dot structures Another way to represent electrons Focuses on the outermost electrons—the ones in the outermost energy level called the valence electrons. Valence e’s are important because: – They determine how an atom reacts chemically. – It is part of how elements are organized on the periodic table.

What are valence electrons? Valence electrons are the ones in the outermost ring—the number to the right.

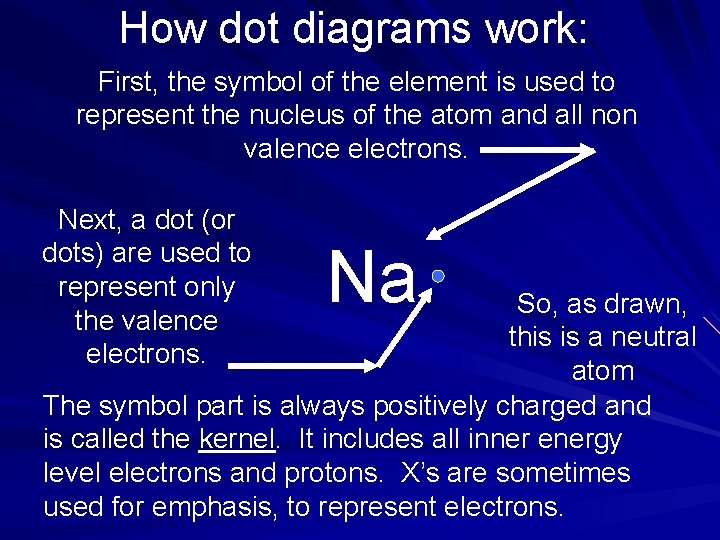
How dot diagrams work: First, the symbol of the element is used to represent the nucleus of the atom and all non valence electrons. Next, a dot (or dots) are used to represent only the valence electrons. Na So, as drawn, this is a neutral atom The symbol part is always positively charged and is called the kernel. It includes all inner energy level electrons and protons. X’s are sometimes used for emphasis, to represent electrons.

Arrangement of the dots: Some students get hung up on this. The convention says to start with the first two on “top” at the twelve o’clock position. However, as you will see some variations are allowed depending on whether you are drawing an atom or a bonded atom. For example: C O Cl Al P 2 -4 2 -6 2 -8 -7 2 -8 -3 2 -8 -5 For any element, check the electron configuration. The last number is/are the valence electrons. Add those around the symbol.

End