Drawing Atoms CHEMICAL SCIENCE Review Atomic Structure Subatomic

Drawing Atoms CHEMICAL SCIENCE
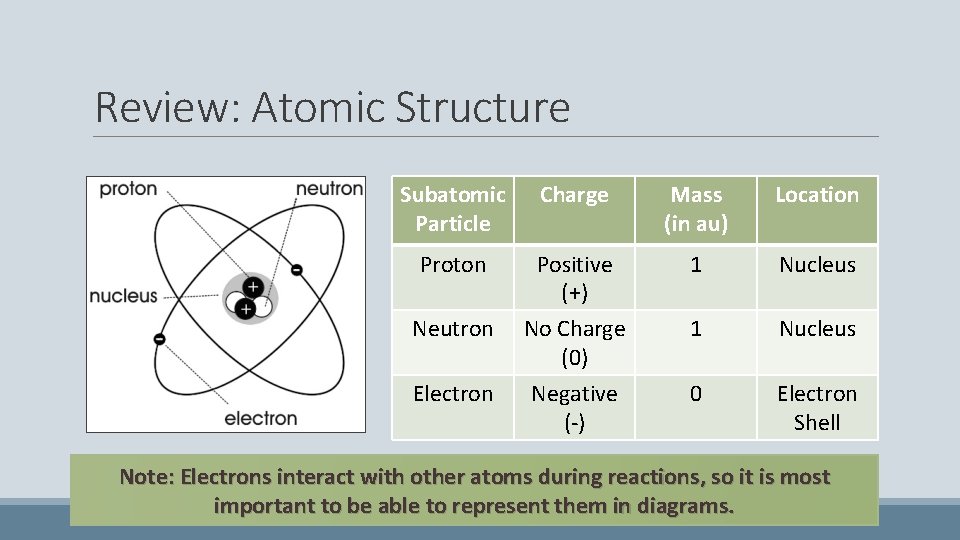
Review: Atomic Structure Subatomic Particle Charge Mass (in au) Location Proton Positive (+) 1 Nucleus Neutron No Charge (0) Negative (-) 1 Nucleus 0 Electron Shell Electron Note: Electrons interact with other atoms during reactions, so it is most important to be able to represent them in diagrams.

Bohr Rutherford Diagrams Bohr Rutherford diagrams show the arrangement of all atoms. To draw one we need to know: 1. How many electrons does this element have? 2. How many electrons go in each shell? 3. How do electrons arrange themselves?
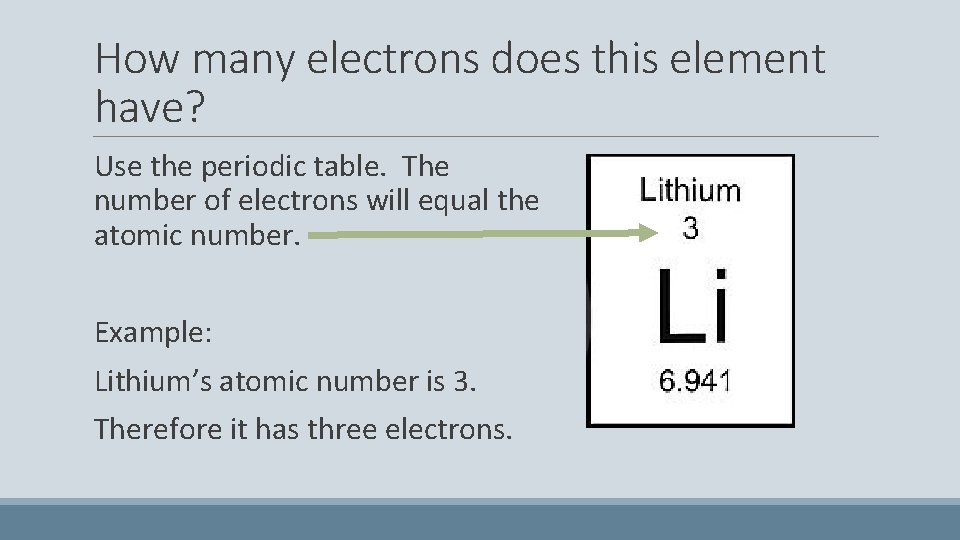
How many electrons does this element have? Use the periodic table. The number of electrons will equal the atomic number. Example: Lithium’s atomic number is 3. Therefore it has three electrons.

Note: Although the third shell can hold 18 electrons, it doesn’t always fill up. The first twenty elements only hold How many electrons go in each shell? 8 electrons in the third shell.
How do electrons arrange themselves? In the first, shell they pair up. After the first shell, they pair up after 4 electrons. Note: This is easier to understand as you go, so let’s look at some examples!
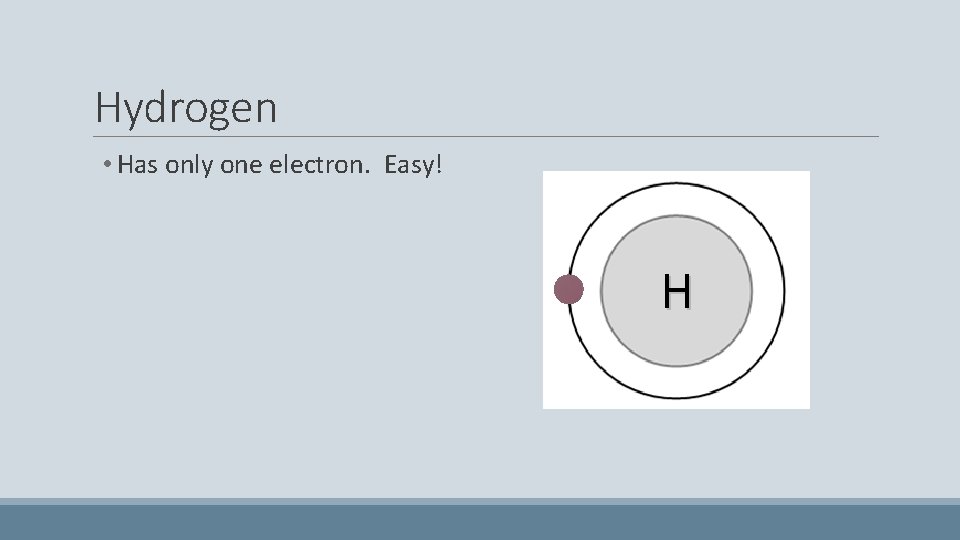
Hydrogen • Has only one electron. Easy! H
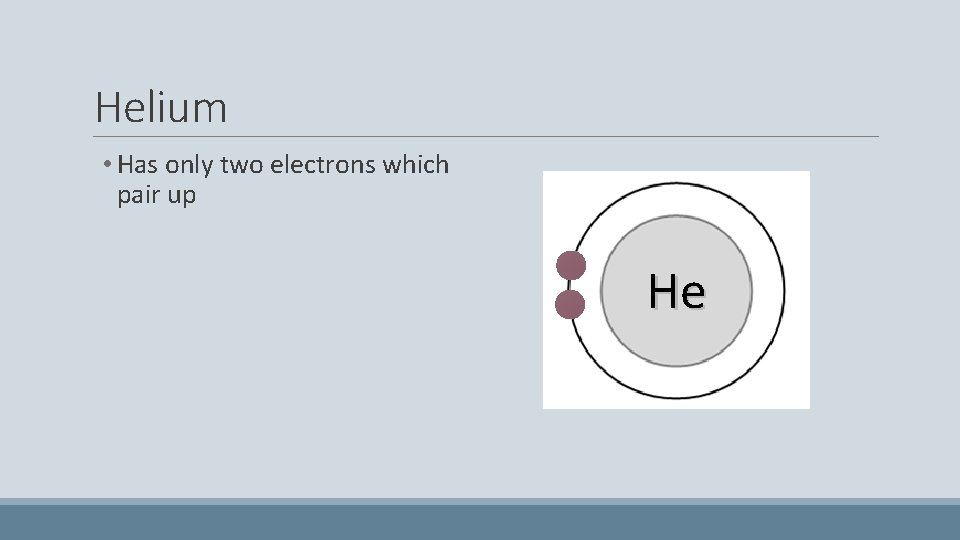
Helium • Has only two electrons which pair up He
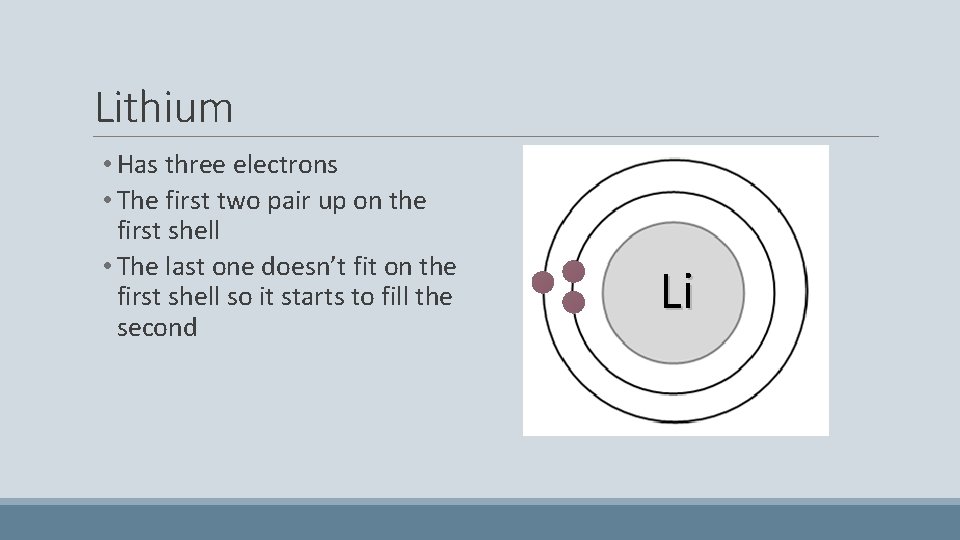
Lithium • Has three electrons • The first two pair up on the first shell • The last one doesn’t fit on the first shell so it starts to fill the second Li
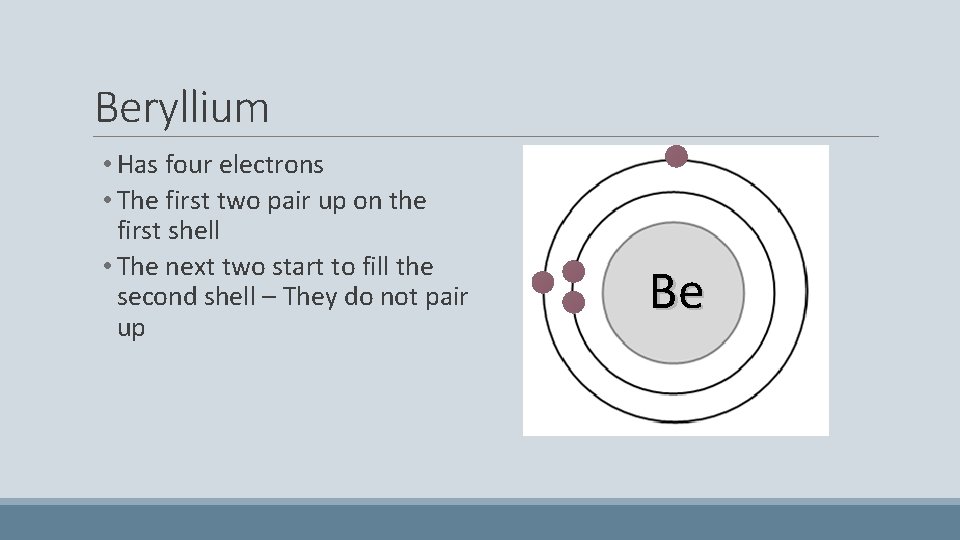
Beryllium • Has four electrons • The first two pair up on the first shell • The next two start to fill the second shell – They do not pair up Be
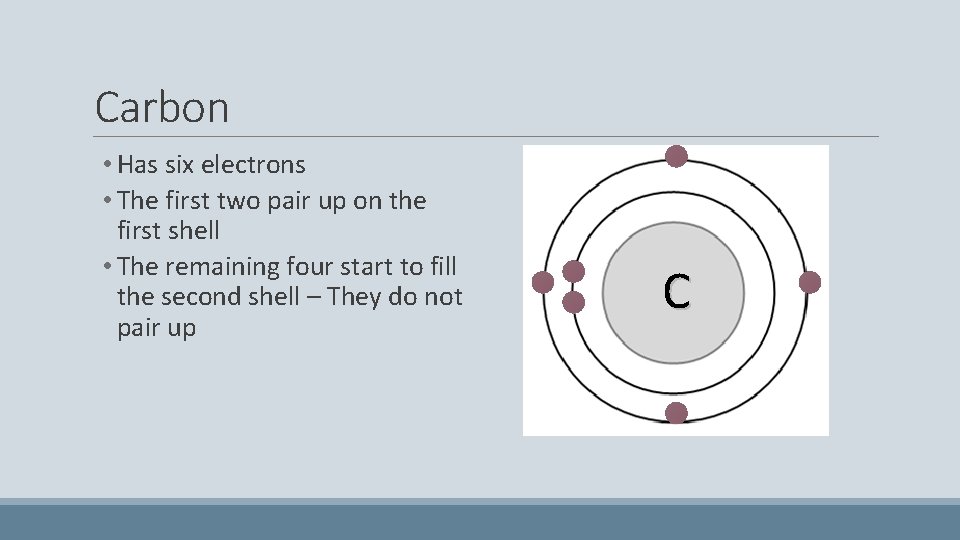
Carbon • Has six electrons • The first two pair up on the first shell • The remaining four start to fill the second shell – They do not pair up C
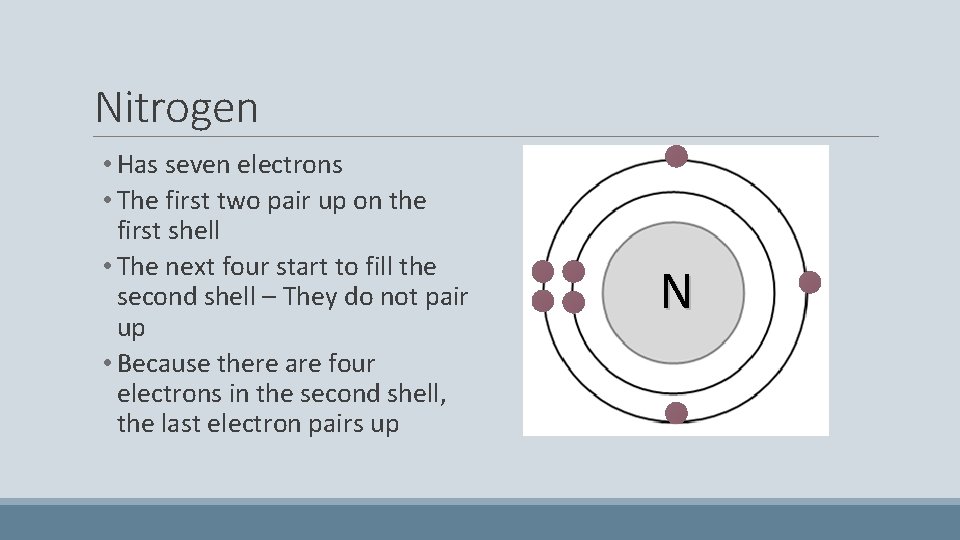
Nitrogen • Has seven electrons • The first two pair up on the first shell • The next four start to fill the second shell – They do not pair up • Because there are four electrons in the second shell, the last electron pairs up N
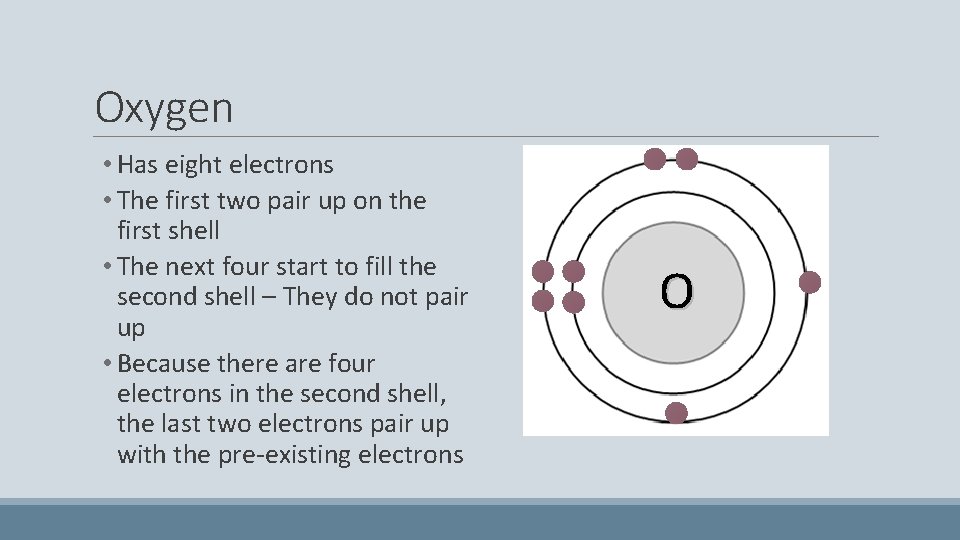
Oxygen • Has eight electrons • The first two pair up on the first shell • The next four start to fill the second shell – They do not pair up • Because there are four electrons in the second shell, the last two electrons pair up with the pre-existing electrons O
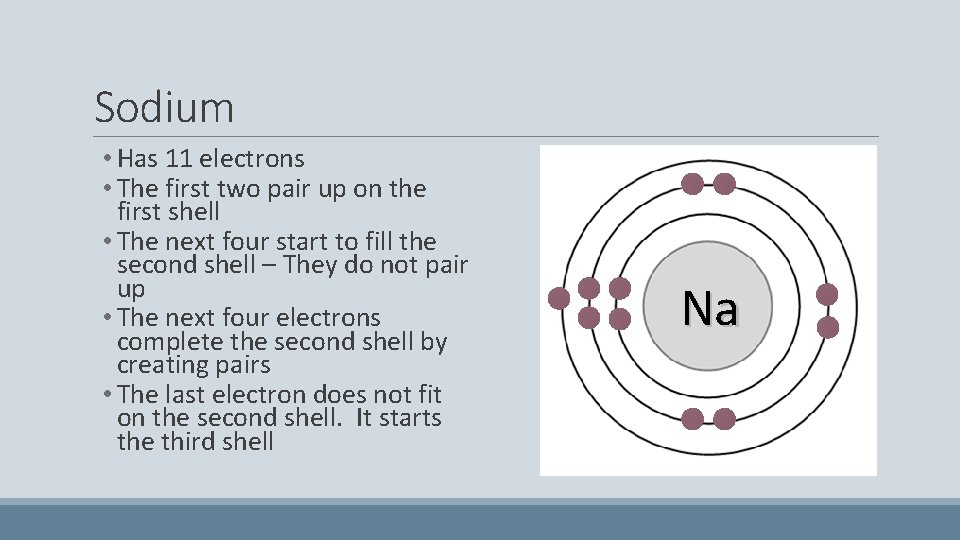
Sodium • Has 11 electrons • The first two pair up on the first shell • The next four start to fill the second shell – They do not pair up • The next four electrons complete the second shell by creating pairs • The last electron does not fit on the second shell. It starts the third shell Na
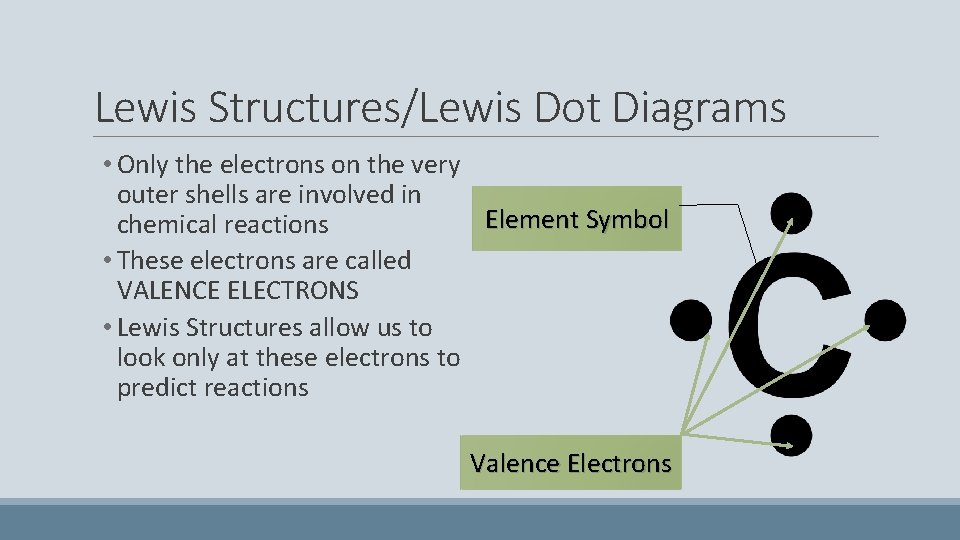
Lewis Structures/Lewis Dot Diagrams • Only the electrons on the very outer shells are involved in Element Symbol chemical reactions • These electrons are called VALENCE ELECTRONS • Lewis Structures allow us to look only at these electrons to predict reactions Valence Electrons
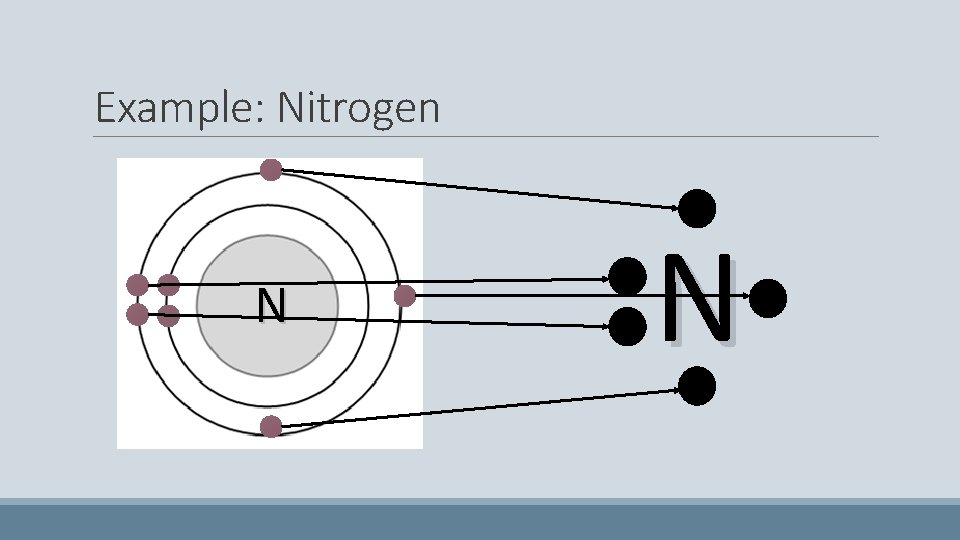
Example: Nitrogen N N
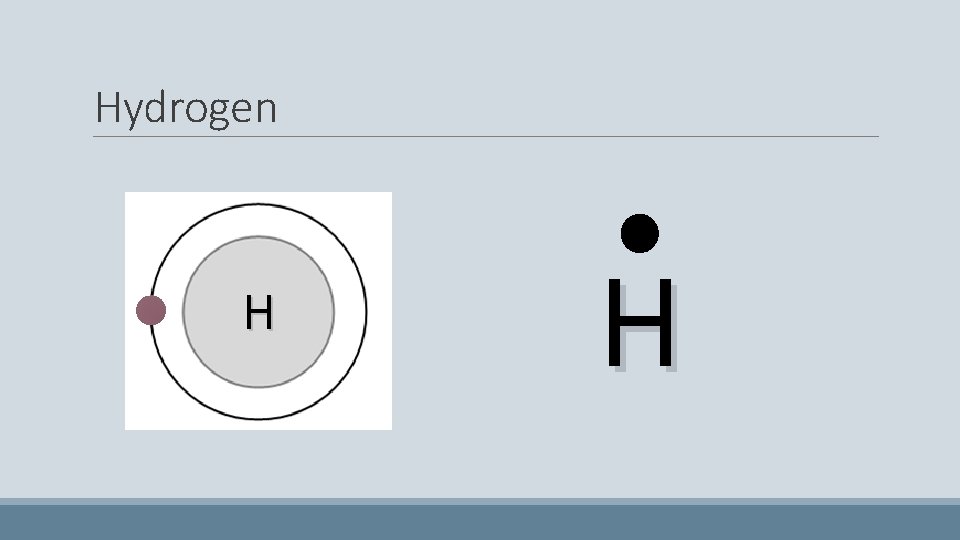
Hydrogen H H
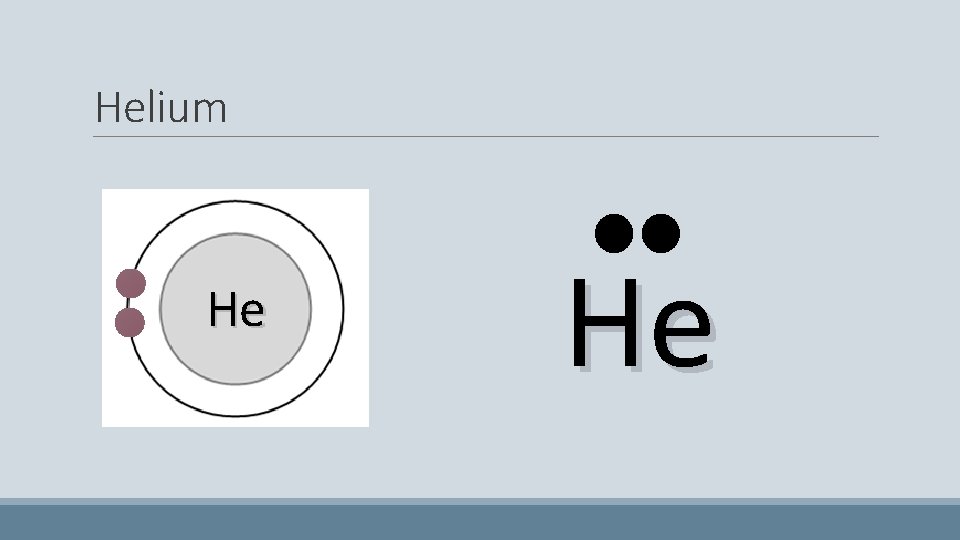
Helium He He
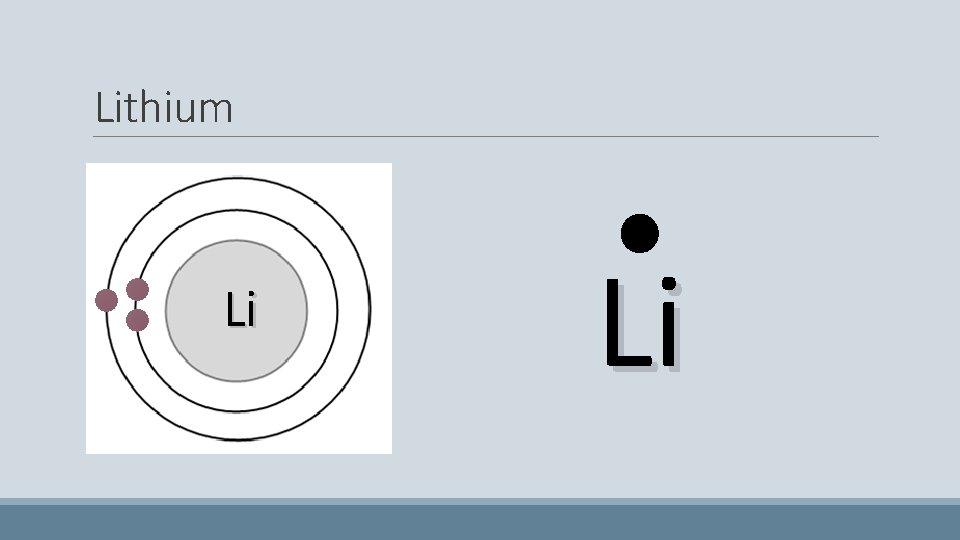
Lithium Li Li
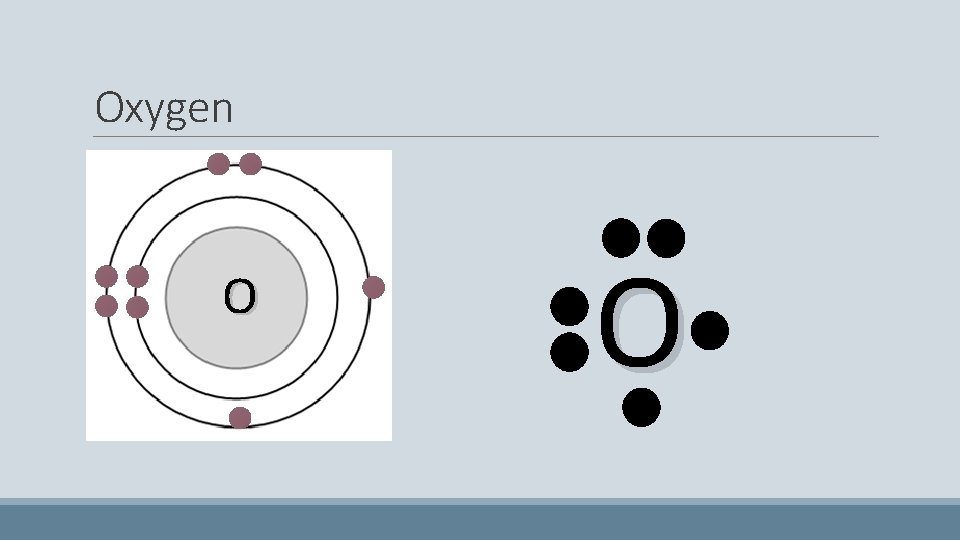
Oxygen O O
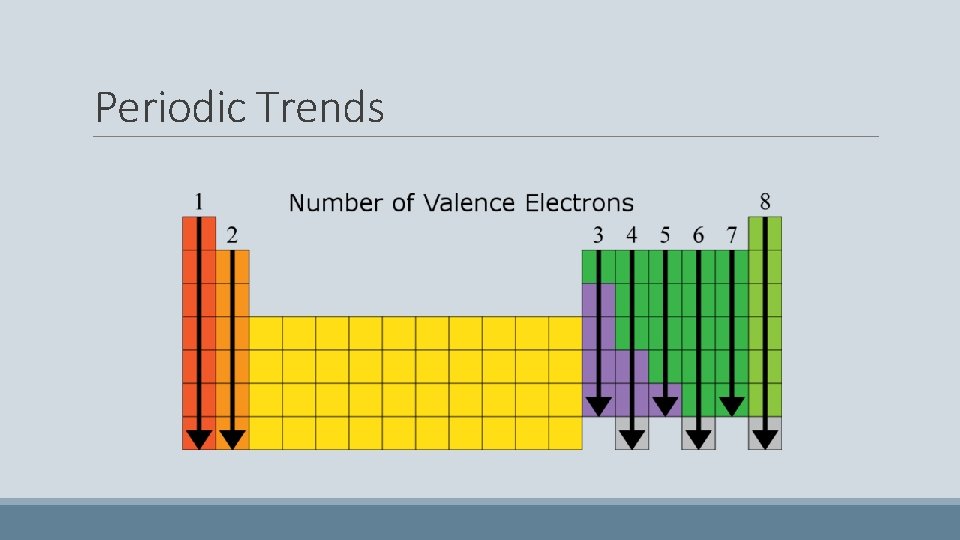
Periodic Trends

Your turn! COMPLETE WORKSHEET
- Slides: 22