Draw the parts of a Hydrogen atom on
- Slides: 13

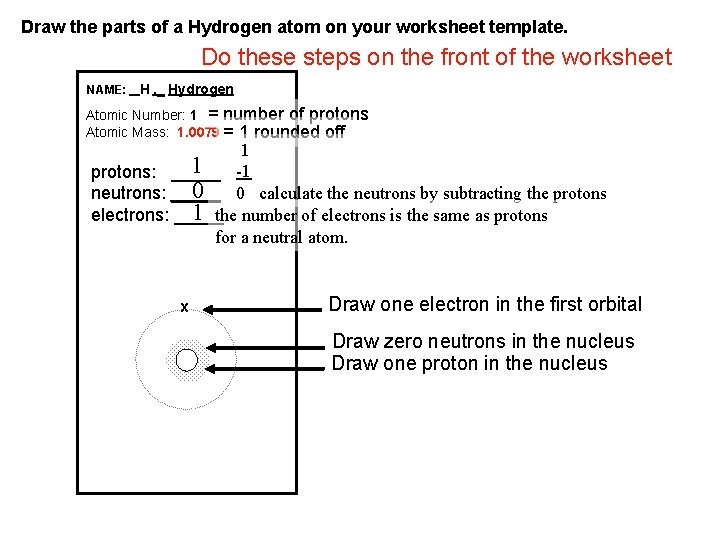
Draw the parts of a Hydrogen atom on your worksheet template. Do these steps on the front of the worksheet NAME: _ H , _ Hydrogen Atomic Number: 1 = number of protons Atomic Mass: 1. 0079 = 1 rounded off 1 1 -1 protons: _____ 0 0 calculate the neutrons by subtracting the protons neutrons: _____ 1 the number of electrons is the same as protons electrons: _____ for a neutral atom. x Draw one electron in the first orbital Draw zero neutrons in the nucleus Draw one proton in the nucleus

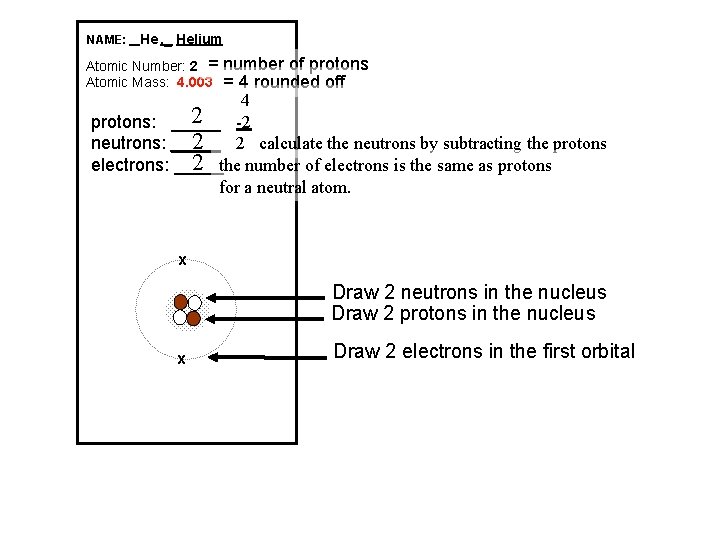
NAME: _ He , _ Helium Atomic Number: 2 = Atomic Mass: 4. 003 number of protons = 4 rounded off 4 2 -2 protons: _____ 2 2 calculate the neutrons by subtracting the protons neutrons: _____ 2 the number of electrons is the same as protons electrons: _____ for a neutral atom. x Draw 2 neutrons in the nucleus Draw 2 protons in the nucleus x Draw 2 electrons in the first orbital

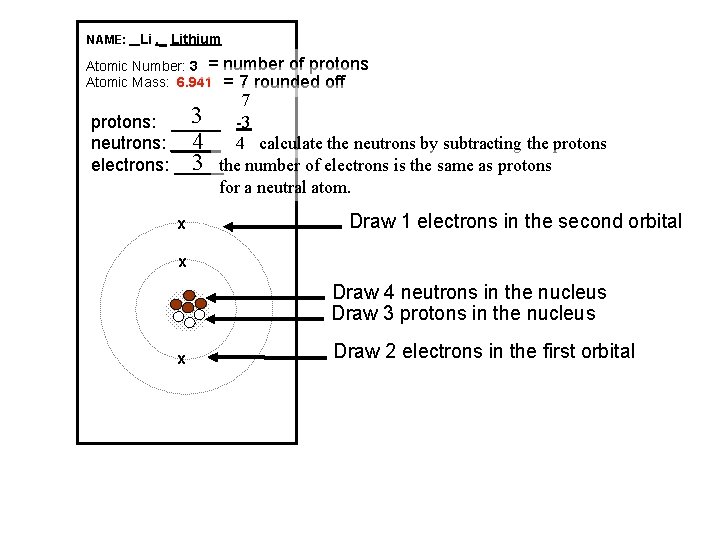
NAME: _ Li , _ Lithium Atomic Number: 3 = Atomic Mass: 6. 941 number of protons = 7 rounded off 7 3 -3 protons: _____ 4 4 calculate the neutrons by subtracting the protons neutrons: _____ 3 the number of electrons is the same as protons electrons: _____ for a neutral atom. x Draw 1 electrons in the second orbital x Draw 4 neutrons in the nucleus Draw 3 protons in the nucleus x Draw 2 electrons in the first orbital

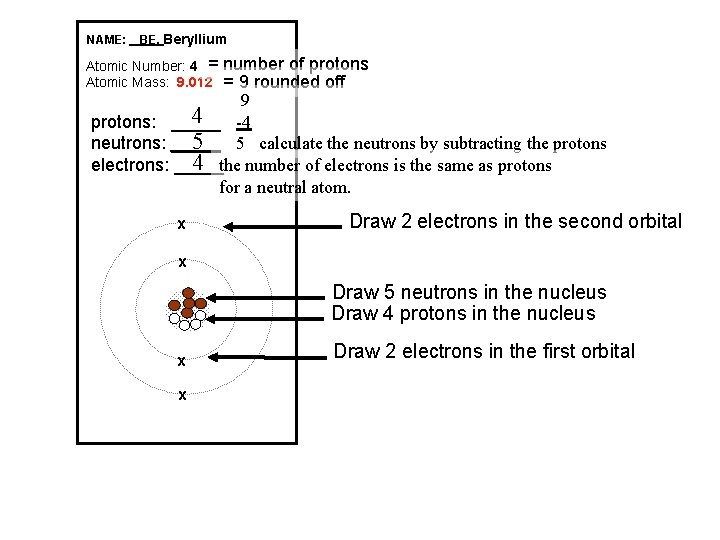
NAME: _ BE, Beryllium Atomic Number: 4 = Atomic Mass: 9. 012 number of protons = 9 rounded off 9 4 -4 protons: _____ 5 5 calculate the neutrons by subtracting the protons neutrons: _____ 4 the number of electrons is the same as protons electrons: _____ for a neutral atom. x Draw 2 electrons in the second orbital x Draw 5 neutrons in the nucleus Draw 4 protons in the nucleus x x Draw 2 electrons in the first orbital

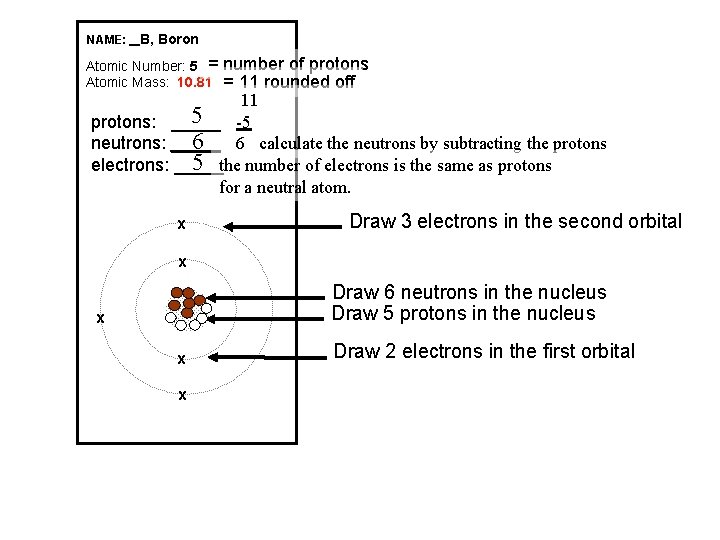
NAME: _ B, Boron Atomic Number: 5 = Atomic Mass: 10. 81 number of protons = 11 rounded off 11 5 -5 protons: _____ 6 6 calculate the neutrons by subtracting the protons neutrons: _____ 5 the number of electrons is the same as protons electrons: _____ for a neutral atom. x Draw 3 electrons in the second orbital x Draw 6 neutrons in the nucleus Draw 5 protons in the nucleus x x x Draw 2 electrons in the first orbital

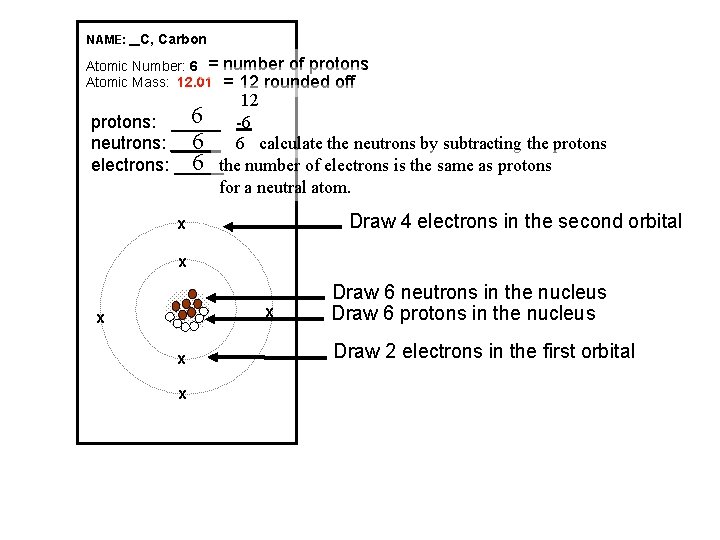
NAME: _ C, Carbon Atomic Number: 6 = Atomic Mass: 12. 01 number of protons = 12 rounded off 12 6 -6 protons: _____ 6 6 calculate the neutrons by subtracting the protons neutrons: _____ 6 the number of electrons is the same as protons electrons: _____ for a neutral atom. Draw 4 electrons in the second orbital x x x Draw 6 neutrons in the nucleus Draw 6 protons in the nucleus Draw 2 electrons in the first orbital

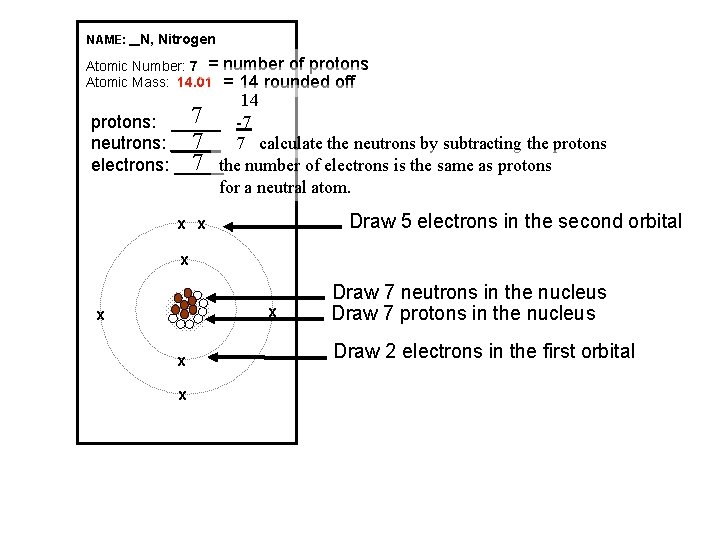
NAME: _ N, Nitrogen Atomic Number: 7 = Atomic Mass: 14. 01 number of protons = 14 rounded off 14 7 -7 protons: _____ 7 7 calculate the neutrons by subtracting the protons neutrons: _____ 7 the number of electrons is the same as protons electrons: _____ for a neutral atom. Draw 5 electrons in the second orbital x x x x Draw 7 neutrons in the nucleus Draw 7 protons in the nucleus Draw 2 electrons in the first orbital

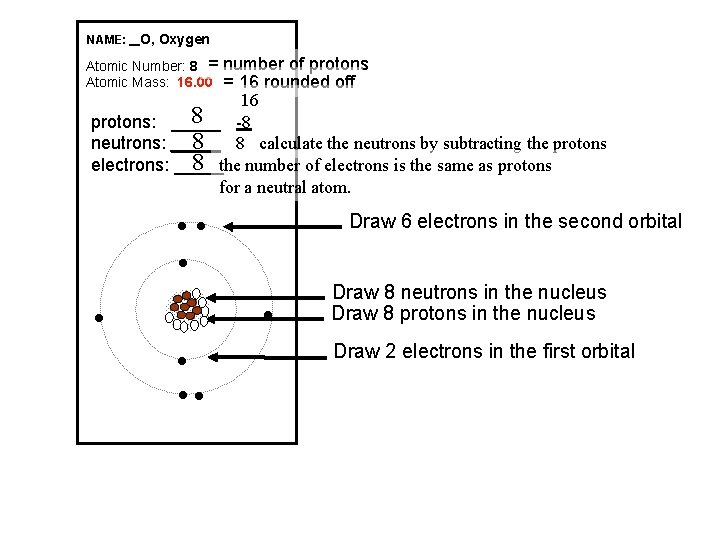
NAME: _ O, Oxygen Atomic Number: 8 = Atomic Mass: 16. 00 number of protons = 16 rounded off 16 8 -8 protons: _____ 8 8 calculate the neutrons by subtracting the protons neutrons: _____ 8 the number of electrons is the same as protons electrons: _____ for a neutral atom. Draw 6 electrons in the second orbital l l l l Draw 8 neutrons in the nucleus Draw 8 protons in the nucleus Draw 2 electrons in the first orbital

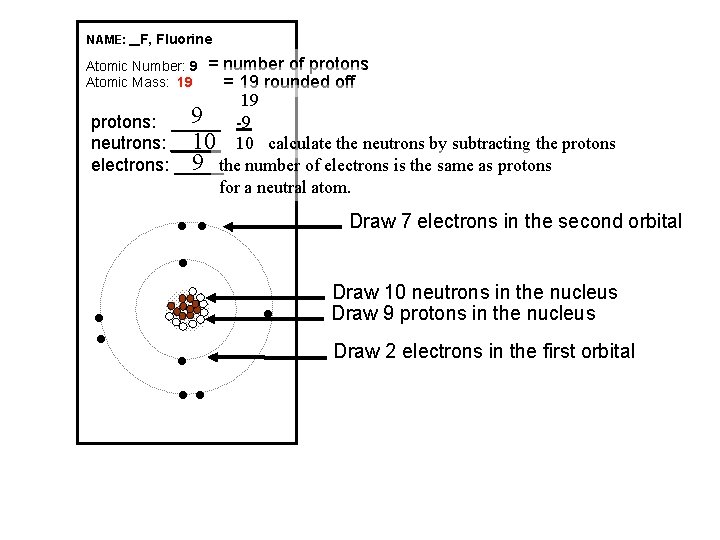
NAME: _ F, Fluorine = number of protons = 19 rounded off 19 9 -9 protons: _____ 10 10 calculate the neutrons by subtracting the protons neutrons: _____ 9 the number of electrons is the same as protons electrons: _____ for a neutral atom. Atomic Number: 9 Atomic Mass: 19 Draw 7 electrons in the second orbital l l l l Draw 10 neutrons in the nucleus Draw 9 protons in the nucleus Draw 2 electrons in the first orbital

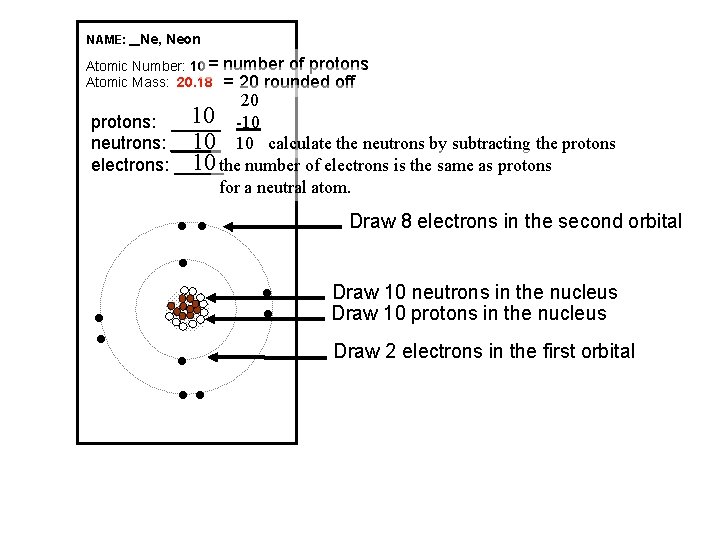
NAME: _ Ne, Neon Atomic Number: 10 = Atomic Mass: 20. 18 number of protons = 20 rounded off 20 10 -10 protons: _____ 10 10 calculate the neutrons by subtracting the protons neutrons: _____ 10 the number of electrons is the same as protons electrons: _____ for a neutral atom. Draw 8 electrons in the second orbital l l Draw 10 neutrons in the nucleus Draw 10 protons in the nucleus Draw 2 electrons in the first orbital

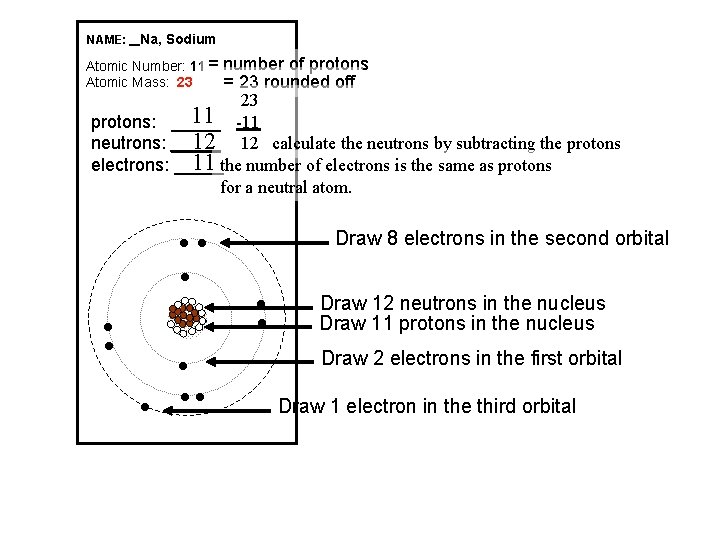
NAME: _ Na, Sodium Atomic Number: 11 = Atomic Mass: 23 number of protons = 23 rounded off 23 11 -11 protons: _____ 12 12 calculate the neutrons by subtracting the protons neutrons: _____ 11 the number of electrons is the same as protons electrons: _____ for a neutral atom. Draw 8 electrons in the second orbital l ll Draw 12 neutrons in the nucleus Draw 11 protons in the nucleus Draw 2 electrons in the first orbital Draw 1 electron in the third orbital

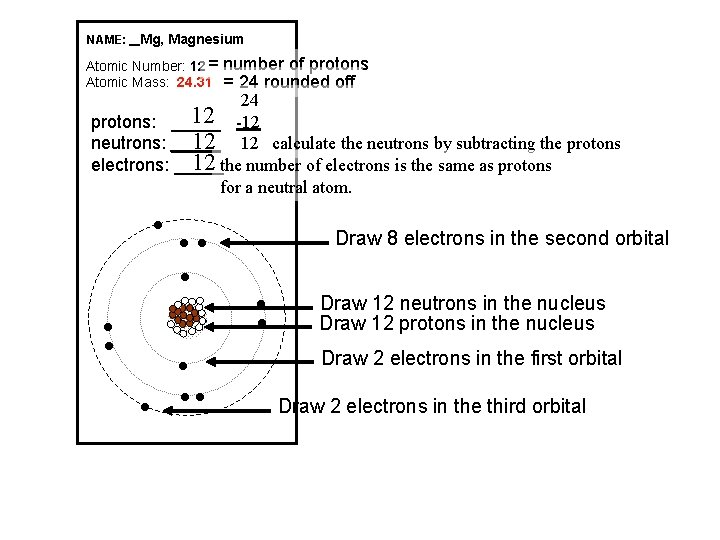
NAME: _ Mg, Magnesium Atomic Number: 12 = Atomic Mass: 24. 31 number of protons = 24 rounded off 24 12 -12 protons: _____ 12 12 calculate the neutrons by subtracting the protons neutrons: _____ 12 the number of electrons is the same as protons electrons: _____ for a neutral atom. l Draw 8 electrons in the second orbital l ll Draw 12 neutrons in the nucleus Draw 12 protons in the nucleus Draw 2 electrons in the first orbital Draw 2 electrons in the third orbital

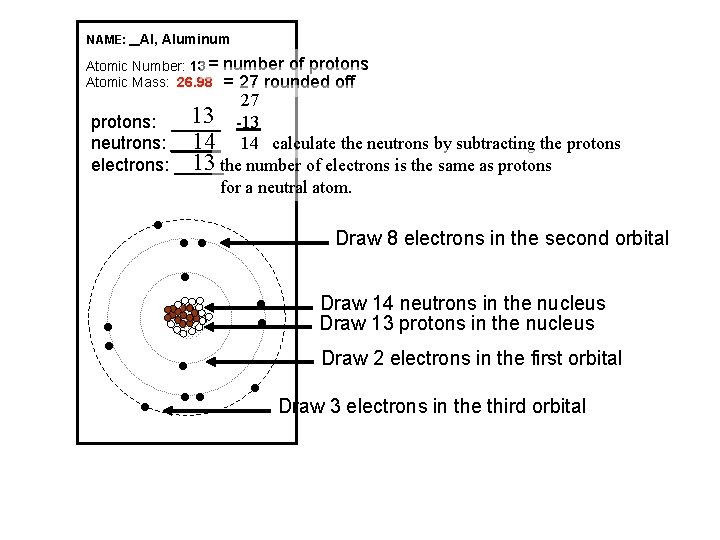
NAME: _ Al, Aluminum Atomic Number: 13 = Atomic Mass: 26. 98 number of protons = 27 rounded off 27 13 -13 protons: _____ 14 14 calculate the neutrons by subtracting the protons neutrons: _____ 13 the number of electrons is the same as protons electrons: _____ for a neutral atom. l Draw 8 electrons in the second orbital l l l Draw 2 electrons in the first orbital l l ll Draw 14 neutrons in the nucleus Draw 13 protons in the nucleus l Draw 3 electrons in the third orbital