Draw the particle arrangement for the states of

















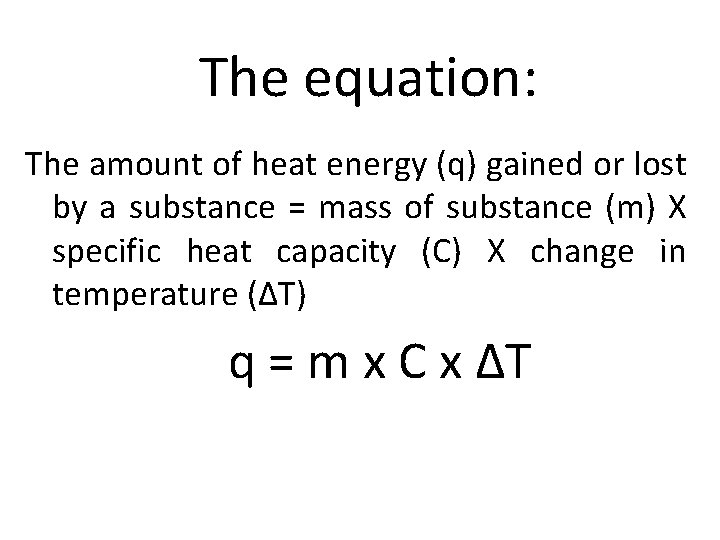

- Slides: 31


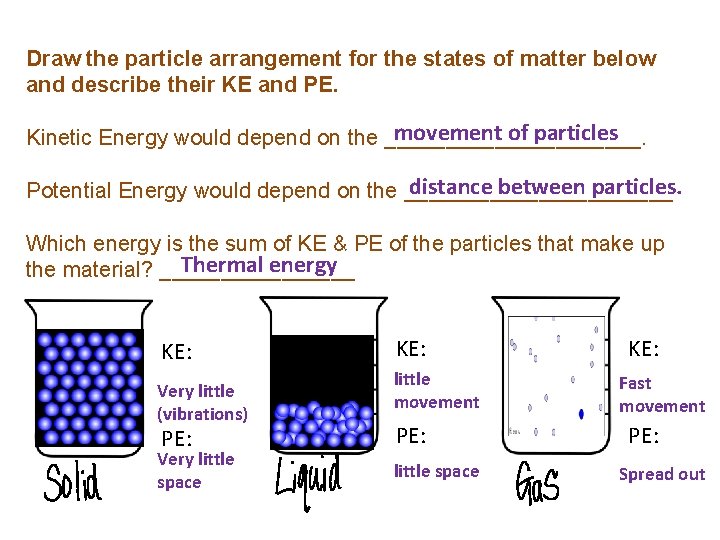
Draw the particle arrangement for the states of matter below and describe their KE and PE. movement of particles Kinetic Energy would depend on the ___________. distance between particles. Potential Energy would depend on the ___________ Which energy is the sum of KE & PE of the particles that make up Thermal energy the material? ________ KE: Very little (vibrations) PE: Very little space KE: little movement PE: little space KE: Fast movement PE: Spread out

Objective 1 • Today’s objective is to understand the difference between thermal energy and temperature.

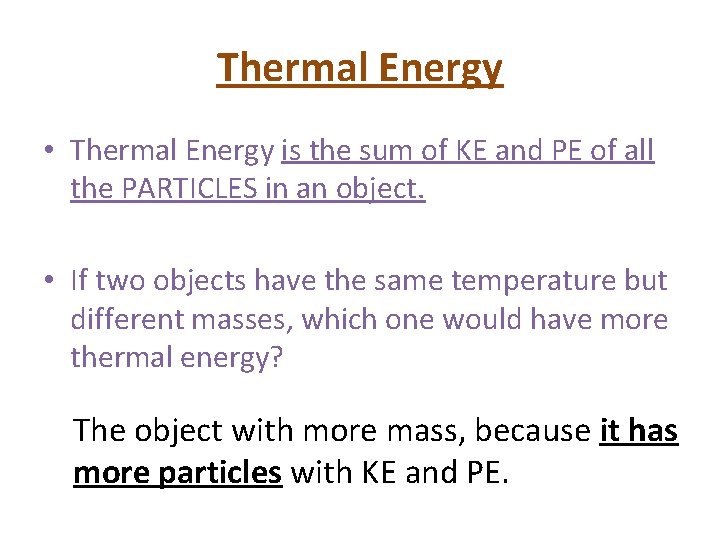
Thermal Energy • Thermal Energy is the sum of KE and PE of all the PARTICLES in an object. • If two objects have the same temperature but different masses, which one would have more thermal energy? The object with more mass, because it has more particles with KE and PE.

Practice Which one has more thermal energy …. – A 50° C pot of soup or a 50° C bowl of soup Pot of soup, more mass, more particles – A glass of room temperature water or a gallon of room temperature water A gallon of water, more mass, more particles – A 5° C pitcher of lemonade or a 5° cup of A pitcher of lemonade, more mass, more lemonade particles

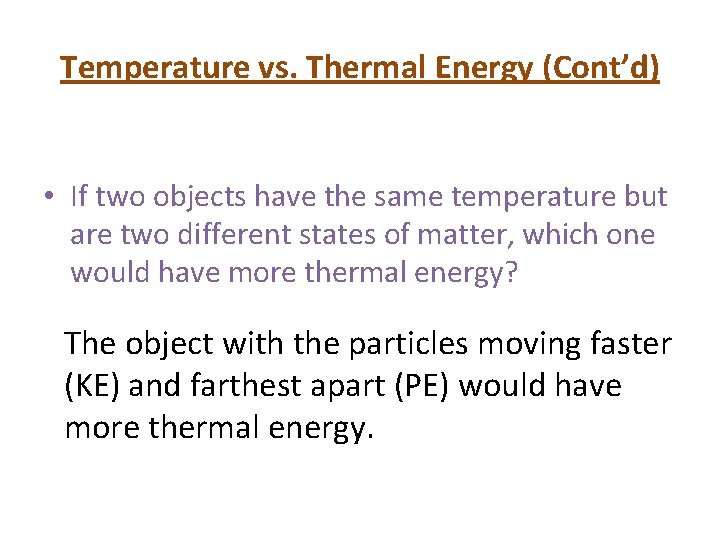
Temperature vs. Thermal Energy (Cont’d) • If two objects have the same temperature but are two different states of matter, which one would have more thermal energy? The object with the particles moving faster (KE) and farthest apart (PE) would have more thermal energy.

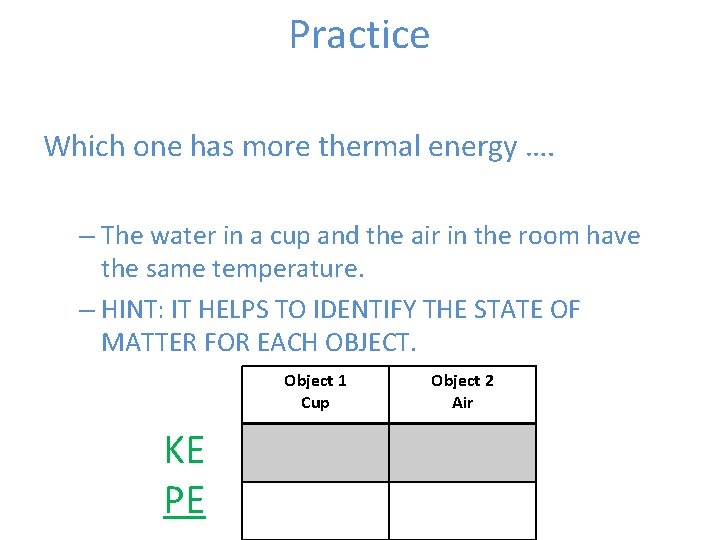
Practice Which one has more thermal energy …. – The water in a cup and the air in the room have the same temperature. – HINT: IT HELPS TO IDENTIFY THE STATE OF MATTER FOR EACH OBJECT. Object 1 Cup KE PE Object 2 Air

Practice Continued • While boiling water you realize the temperature of the metal pot and water is the same. Which one has more thermal energy? Water (liquid) has more distance between particles compared to solid, even though the particles are moving at the same rate. • On a cold windy day, you touched the railing and realized the temperature of the railing and the air is the same. Which one has more thermal energy? Air (gas), has more space between particles, even though the particles are moving at the same rate.

Temperature • Temperature is the measure of the average kinetic energy of the particles in an object. MORE the • The more particle movement, the _______ temperature. The less particle movement, the LESSthe temperature ______ DIRECT • (_____ Relationship)

What type of relationship do temperature and thermal energy have? • Since thermal energy is the sum of KE+PE, when the temperature increases so does the KE. This will result in more thermal energy.

Objective 2 • Today’s objective is to understand the how temperature and the total amount of energy in a system depends on the types, states and amounts of matter present.

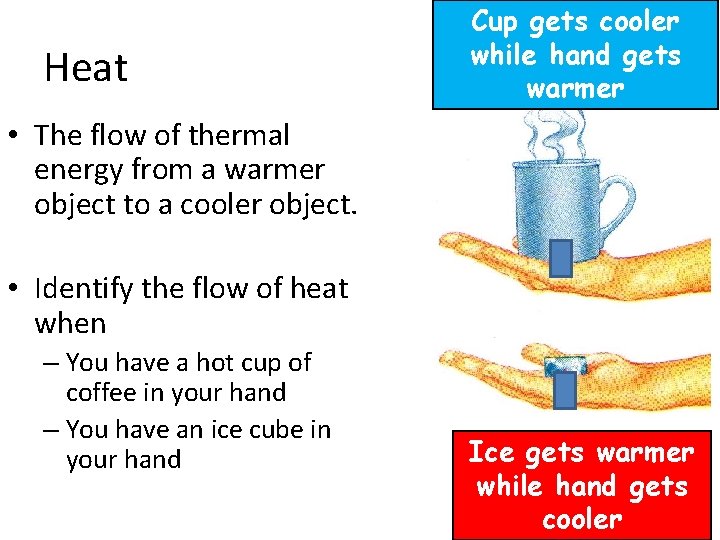
Heat Cup gets cooler while hand gets warmer • The flow of thermal energy from a warmer object to a cooler object. • Identify the flow of heat when – You have a hot cup of coffee in your hand – You have an ice cube in your hand Ice gets warmer while hand gets cooler

Develop a CLAIM • Discuss with your table and be ready to report out to the class: – What do you think will happen when we place each balloon (one blown up with water, one blown up with air only) over fire? • You have 2 minutes to discuss and write your claim in your notebook

Observe and Analyze – What did you observe? • Balloon 1: • Balloon 2: – Was your claim correct? Use evidence to explain. The claim was correct/incorrect because… – Why did this occur? The water (with a higher specific heat than air) allowed the heat from the flame to be absorbed by the water. – What do you think would eventually happen if we continue to leave the standing balloon over the fire? Why?

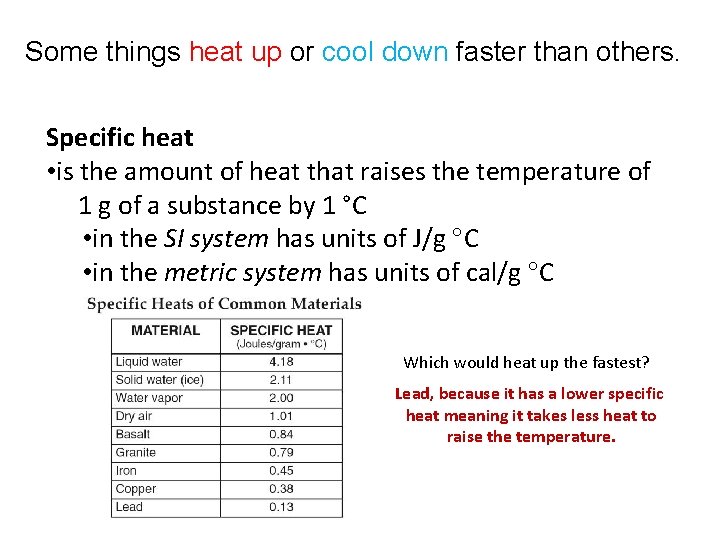
Some things heat up or cool down faster than others. Specific heat • is the amount of heat that raises the temperature of 1 g of a substance by 1 °C • Land in theheats SI system has units of J/gfaster C than water up and cools down • in the metric system has units of cal/g C Which would heat up the fastest? Lead, because it has a lower specific heat meaning it takes less heat to raise the temperature.

• When the specific heat of an object is high it takes a large amount of energy to: – Increase & decrease temperature • Example: – Much of your body is made of water. Water has a high specific heat that helps prevent your body from overheating. SPECIFIC HEAT

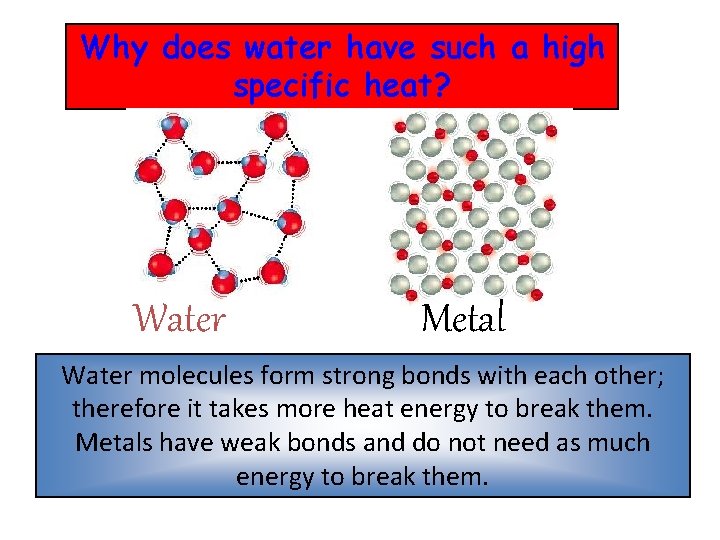
Why does water have such a high specific heat? Water Metal Water molecules form strong bonds with each other; therefore it takes more heat energy to break them. Metals have weak bonds and do not need as much energy to break them.

Scenario Example • Danny put his jeans in the dryer and when he went to get them out and fold them he burned his finger on the metal button. Use specific heat to explain why this occurred. Because the earring is a metal it is a good conductor of heat. Conductors have a lower specific heat so it will heat up quicker.

SCENARIO 1 • When Jane got into her car on a hot summer day – the seat belt buckle burned her leg, but the cloth of the seatbelt did not hurt when she grabbed the seat belt to put it on. Using the term specific heat – identify why this occurred. The specific heat of the metal was lower, therefore it heat up a lot quicker than the cloth. Cloth had a higher specific heat than the metal.

SCENARIO 2 • It was August and Johnny was excited that he finally got a chance to go to the beach. He took off his sandals and stepped on the sand his feet were burning up! He ran right into the ocean and realized that even though the sand was hot the water was fairly cold. Using the term specific heat – explain why this occurred. The specific heat of the sand was lower, therefore it heat up a lot quicker than the water. Water had a higher specific heat than the sand because it was such a large body of water it will take a lot longer to heat up.

SCENARIO 3 • When Catherine was cooking spaghetti she put the pot on the smaller burner on top of the stove. After ten minutes she realized the water still was not boiling and put her finger in the water to test the temperature. After feeling that the water still has not heated up she decided to switch burners. She went to lift the pot and accidently touched the metal and got burned. Using the term specific heat – explained why this occurred. The specific heat of the metal pot was lower, therefore it heat up a lot quicker than the water. Water had a higher specific heat than the metal.

SCENARIO 4 • Mikey ordered pizza for dinner on Friday night. When he opened the box he picked the pizza up from the crust which felt cool and took a bite. As he bit down the roof of his mouth was burning because the sauce was extremely hot. Using the term specific heat explain why this happened. When in the oven for long enough both the crust and sauce reached the same temperature. Due to the fact that the sauce is a liquid it had a higher specific heat than the crust, therefore it took longer to cool down.

SCENARIO 5 • Molly cooked a cherry pie at 200 degrees Celsius. The aluminum film that covered the pie can be touched soon after it is removed while the cherry pie is still dangerously hot. Using specific heat explain why this occurs. When in the oven for long enough both the pie and aluminum reached the same temperature. Due to the fact that the aluminum has a lower specific heat than the pie it will cool down quicker.

SCENARIO 6 • If you were going to replace water as the coolant in an engine, would you look for something with a higher or lower specific heat? Explain You would look for something with a higher specific heat because you Don’t want the car to overheat. If it has a higher specific heat it will take longer to heat up.

SCENARIO 7 • Kari took a spoon out of the kitchen drawer to stir the milk into her coffee. She noticed her coffee was scolding hot when the metal spoon rapidly rose in temperature. Using specific heat explain why this occurred. Because the spoon is a metal it is a good conductor of heat. Conductors have a lower specific heat so it will heat up quicker.

SCENARIO 8 • Jessica was blow drying her hair while wearing metal earrings. She ran her hand through her hair but got burned by the metal earring. Use specific heat to explain why this occurred. Because the earring is a metal it is a good conductor of heat. Conductors have a lower specific heat so it will heat up quicker.
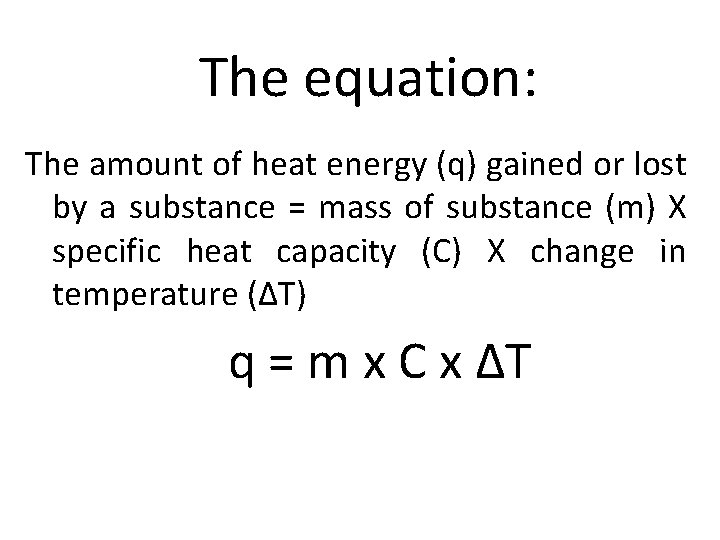
The equation: The amount of heat energy (q) gained or lost by a substance = mass of substance (m) X specific heat capacity (C) X change in temperature (ΔT) q = m x C x ΔT

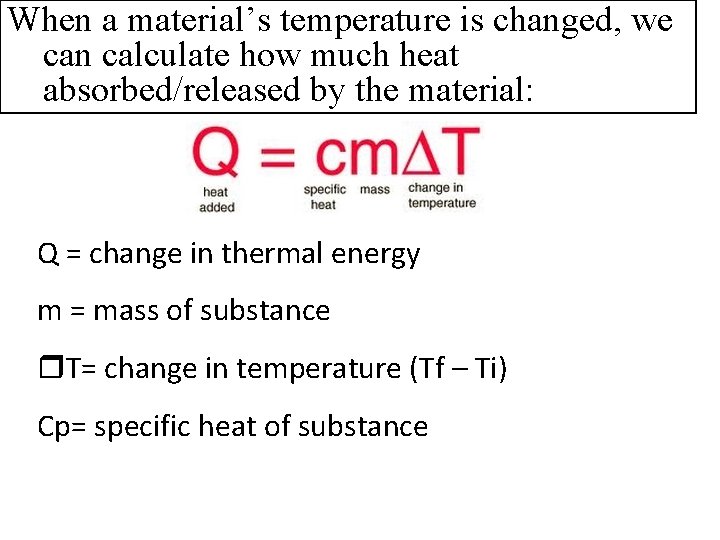
When a material’s temperature is changed, we can calculate how much heat absorbed/released by the material: Q = change in thermal energy m = mass of substance T= change in temperature (Tf – Ti) Cp= specific heat of substance

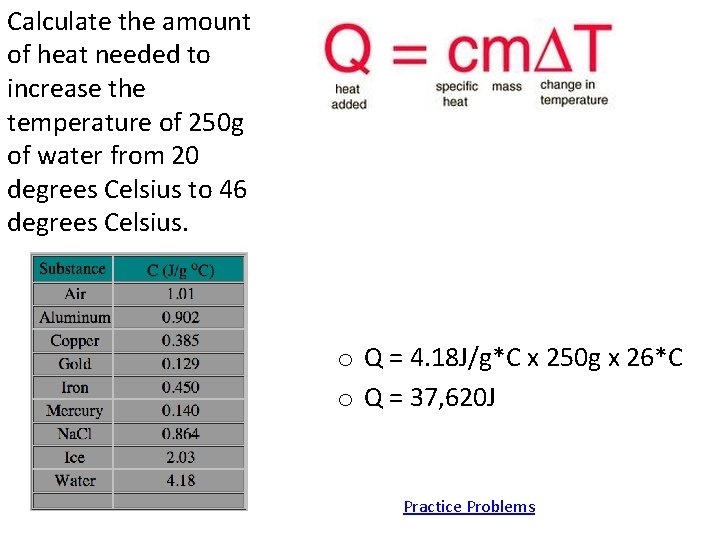
Calculate the amount of heat needed to increase the temperature of 250 g of water from 20 degrees Celsius to 46 degrees Celsius. o Q = 4. 18 J/g*C x 250 g x 26*C o Q = 37, 620 J Practice Problems

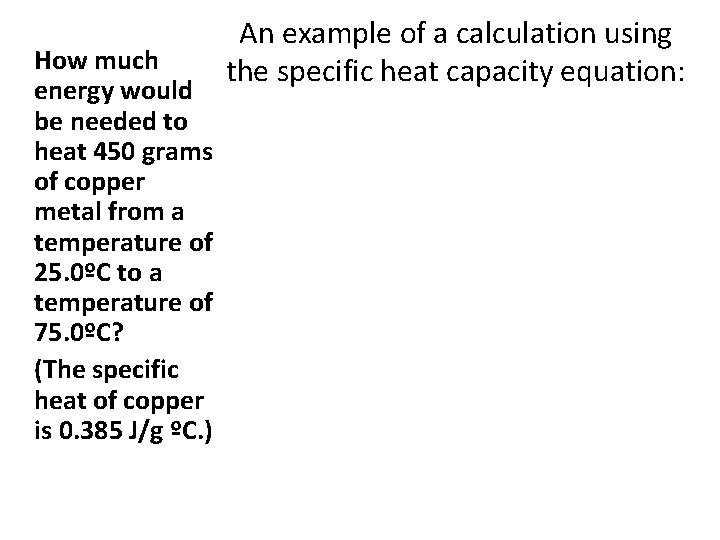
How much energy would be needed to heat 450 grams of copper metal from a temperature of 25. 0ºC to a temperature of 75. 0ºC? (The specific heat of copper is 0. 385 J/g ºC. ) An example of a calculation using the specific heat capacity equation:

Example: How much heat must be absorbed by 375 grams of water to raise its temperature by 25° C? (c water = 4. 18 J/g C)