Draw Lewis Structures for the following compounds Give

































- Slides: 53

• Draw Lewis Structures for the following compounds. Give the shape, polarity, and bond angle for each compound. • CH 3 OH • NH 3 • N 2 H 2

Chapters 7 and 8 Review

Chemical Bonds • electrical attraction between nuclei and valence e- of neighboring atoms that binds the atoms together • bonds form in order to… – decrease potential energy – increase stability • Three types: – Ionic – Covalent – metallic

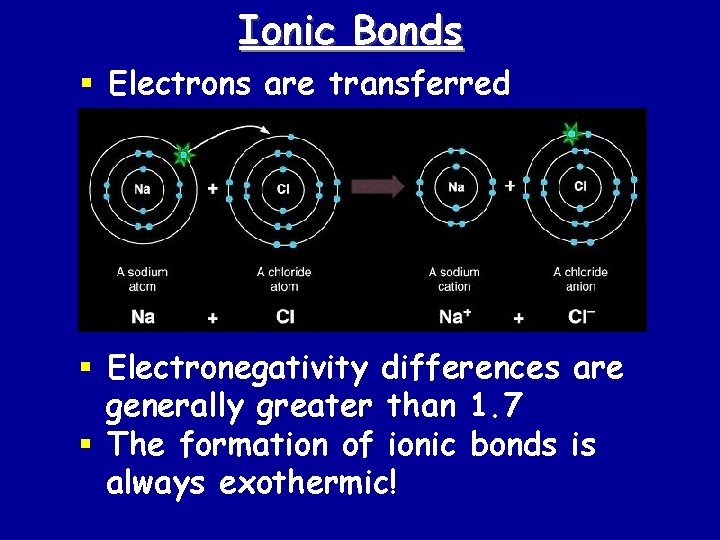
Ionic Bonds § Electrons are transferred § Electronegativity differences are generally greater than 1. 7 § The formation of ionic bonds is always exothermic!

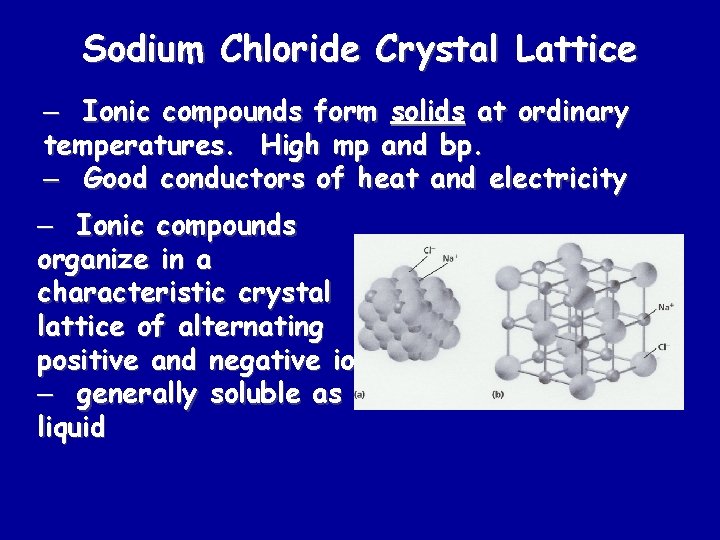
Sodium Chloride Crystal Lattice – Ionic compounds form solids at ordinary temperatures. High mp and bp. – Good conductors of heat and electricity – Ionic compounds organize in a characteristic crystal lattice of alternating positive and negative ions. – generally soluble as a liquid

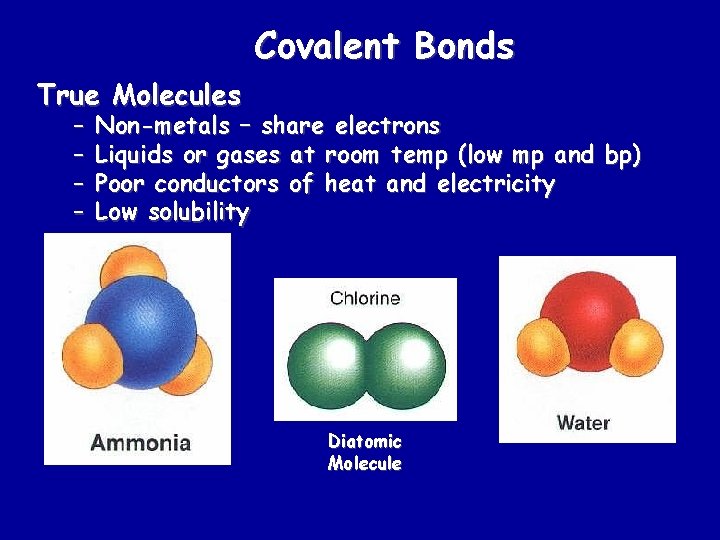
Covalent Bonds True Molecules – – Non-metals – share electrons Liquids or gases at room temp (low mp and bp) Poor conductors of heat and electricity Low solubility Diatomic Molecule

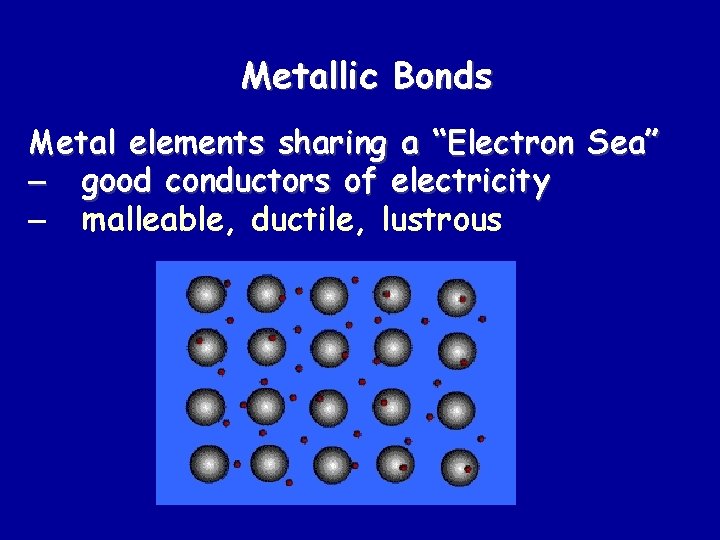
Metallic Bonds Metal elements sharing a “Electron Sea” – good conductors of electricity – malleable, ductile, lustrous

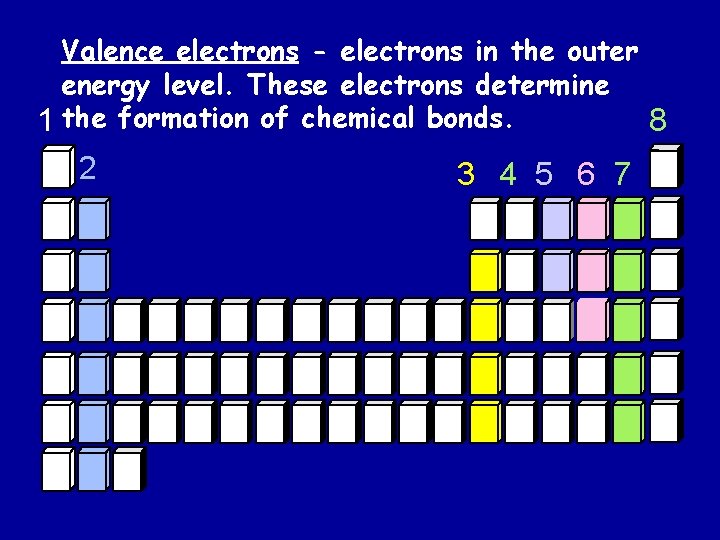
Valence electrons - electrons in the outer energy level. These electrons determine 1 the formation of chemical bonds. 8 2 3 4 5 6 7

• Electronegativity – a measure of an atom’s ability to attract electrons. – higher e- neg atom – lower e- neg atom +

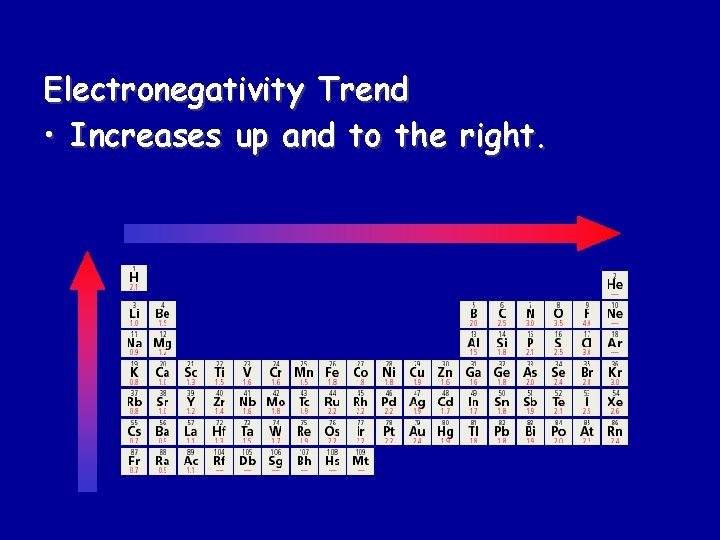
Electronegativity Trend • Increases up and to the right.

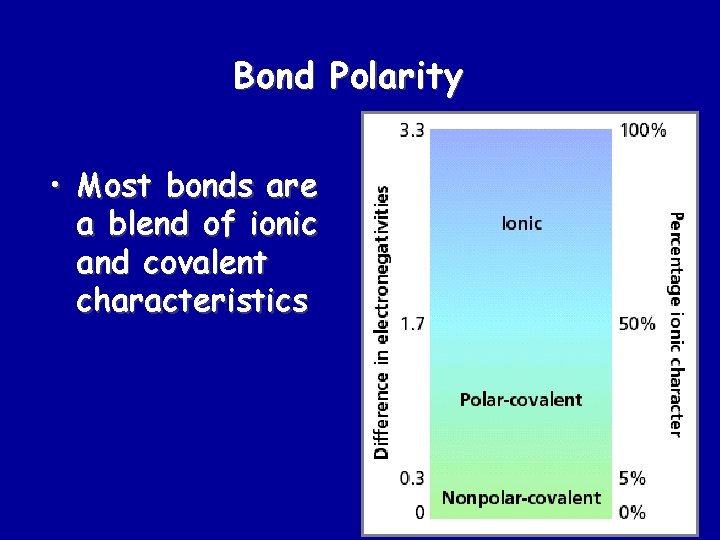
Bond Polarity • Most bonds are a blend of ionic and covalent characteristics

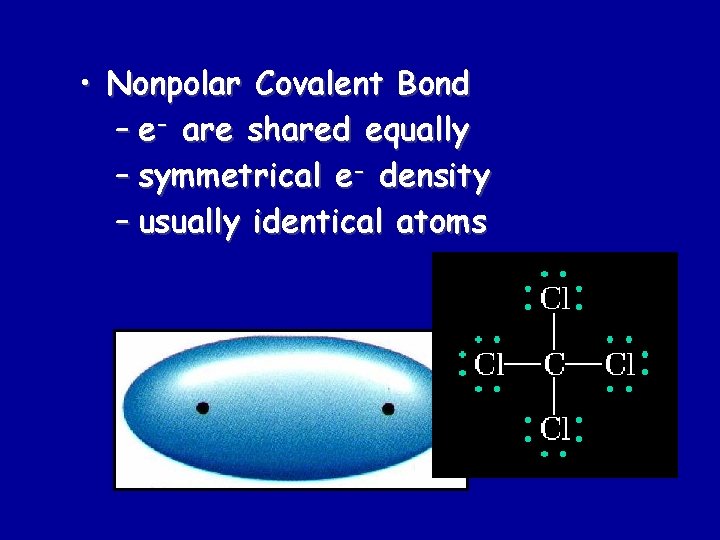
• Nonpolar Covalent Bond – e- are shared equally – symmetrical e- density – usually identical atoms

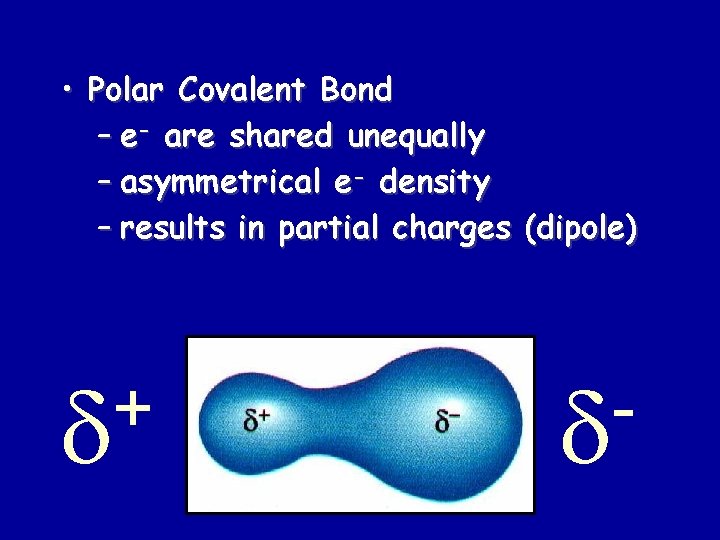
• Polar Covalent Bond – e- are shared unequally – asymmetrical e- density – results in partial charges (dipole) +

Covalent Compounds • Molecules are neutral groups of atoms that are held together by covalent bonds. • Diatomic molecules – H 2, N 2, O 2, F 2, Cl 2, Br 2, and I 2. Allotrophs include P 4 and S 8.

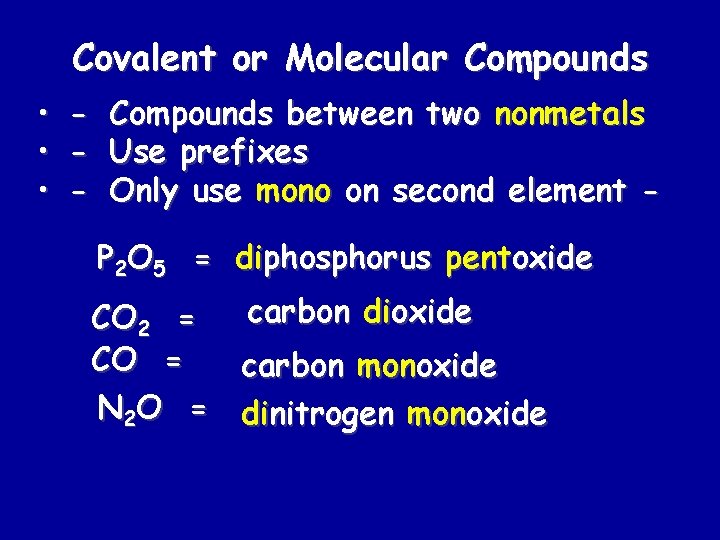
Covalent or Molecular Compounds • • • - Compounds between two nonmetals Use prefixes Only use mono on second element P 2 O 5 = diphosphorus pentoxide carbon dioxide CO 2 = CO = carbon monoxide N 2 O = dinitrogen monoxide

Octet Rule • Remember… – Most atoms form bonds in order to have 8 valence electrons.

Drawing Lewis Diagrams • Find total # of valence e-. • Arrange atoms - singular atom is usually in the middle. • Form bonds between atoms (2 e-). • Distribute remaining e- to give each atom an octet (recall exceptions). • If there aren’t enough e- to go around, form double or triple bonds.

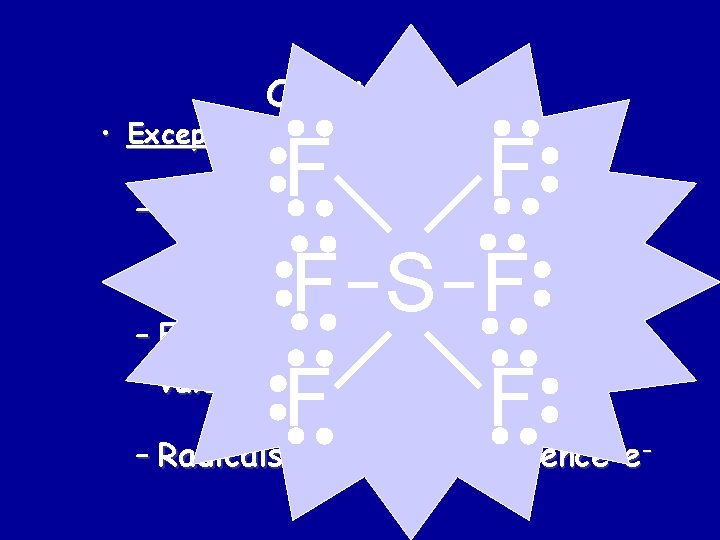
Octet Rule • Exceptions: F F F B F F O SO F H N H F Very unstable!! F F – Hydrogen 2 valence e- – Groups 1, 2, 3 get 2, 4, 6 valence e– Expanded octet more than 8 valence e- (e. g. S, P, Xe) – Radicals odd # of valence e-

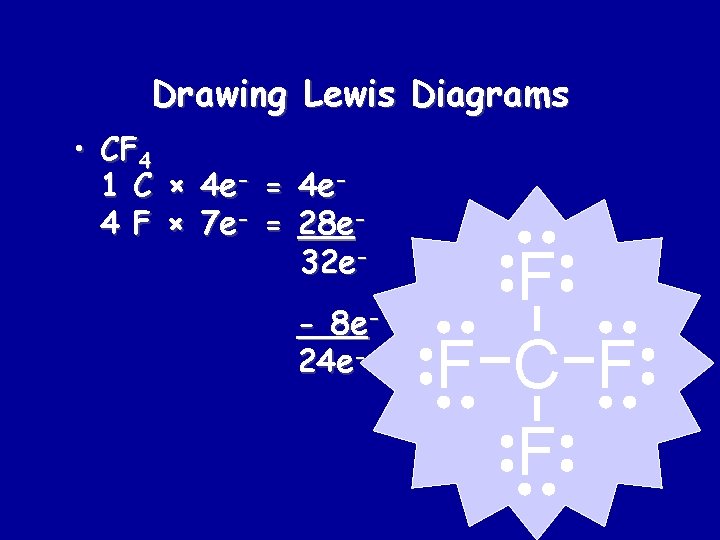
Drawing Lewis Diagrams • CF 4 1 C × 4 e- = 4 e 4 F × 7 e- = 28 e 32 e- 8 e 24 e- F F C F F

Drawing Lewis Diagrams • CO 2 1 C × 4 e- = 4 e 2 O × 6 e- = 12 e 16 e- - 4 e 12 e- O C O

Polyatomic Ions • To find total # of valence e-: – Add 1 e- for each negative charge. – Subtract 1 e- for each positive charge. • Place brackets around the ion and label the charge.

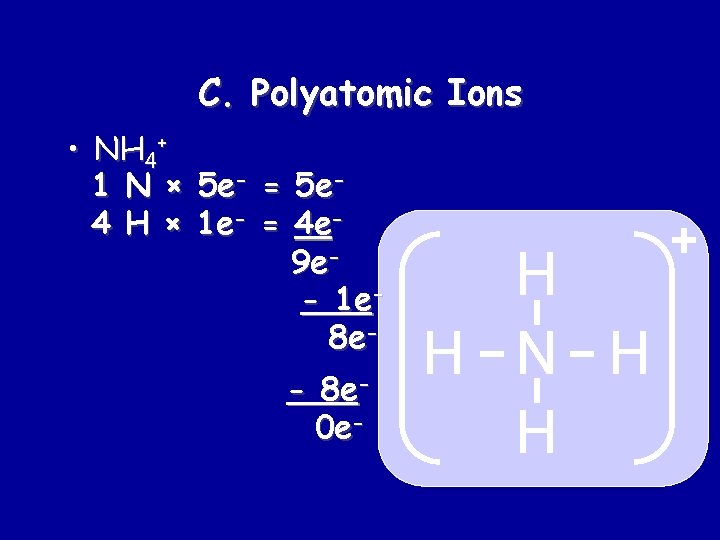
C. Polyatomic Ions • NH 4+ 1 N × 5 e- = 5 e 4 H × 1 e- = 4 e 9 e- 1 e 8 e- 8 e 0 e- H H N H H

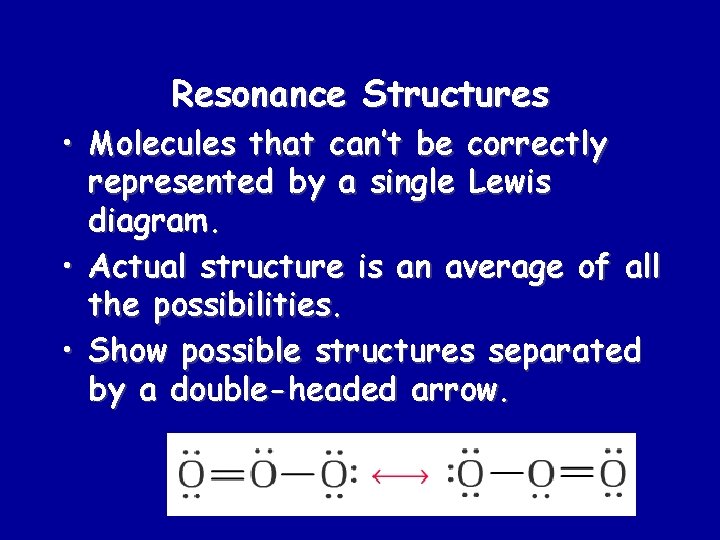
Resonance Structures • Molecules that can’t be correctly represented by a single Lewis diagram. • Actual structure is an average of all the possibilities. • Show possible structures separated by a double-headed arrow.

VSEPR Geometry Zumdahl, De. Coste, World of Chemistry 2002, page 389

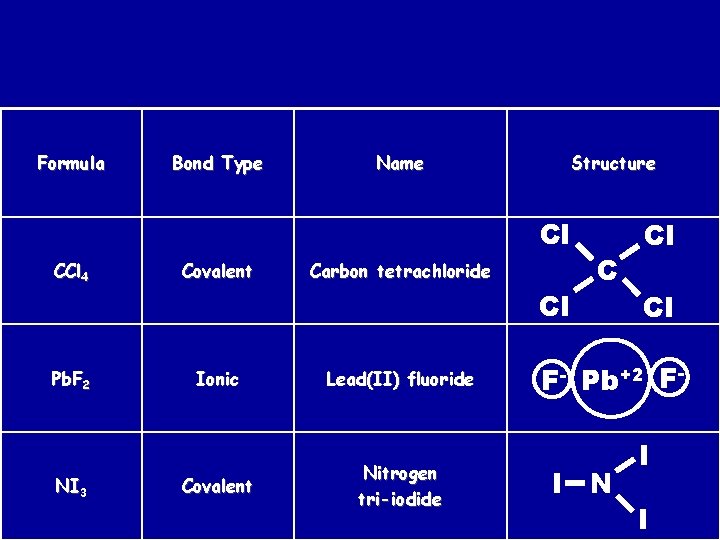
Formula Bond Type Name Structure Cl CCl 4 Covalent Carbon tetrachloride Cl Pb. F 2 NI 3 Ionic Lead(II) fluoride Covalent Nitrogen tri-iodide C Cl Cl F- Pb+2 FI N I I

Ionic Bonding: Force of attraction between oppositely charged ions. Ions • Cation: A positive ion • Mg 2+, NH 4+ • Anion: A negative ion • Cl-, SO 42 -

+1 +2 +3 -3 -2 -1

Writing Ionic Compound Formulas Example: Barium nitrate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 2+ ( Ba NO 3 ) 2 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. Not balanced!

Writing Ionic Compound Formulas Example: Ammonium sulfate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. ( NH 4+) SO 42 - 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. 2 Not balanced!

Writing Ionic Compound Formulas Example: Iron(III) chloride 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. Fe 3+ Cl 3 3 Not balanced!

Writing Ionic Compound Formulas Example: Aluminum sulfide 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. 3+ Al 2 2 S 3 Not balanced!

Writing Ionic Compound Formulas Example: Magnesium carbonate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. Mg 2+ CO 32 They are balanced!

Writing Ionic Compound Formulas Example: Zinc hydroxide 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 2+ ( Zn OH ) 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. 2 Not balanced!

Writing Ionic Compound Formulas Example: Aluminum phosphate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3+ Al 3 PO 4 They ARE balanced!

Naming Ionic Compounds • 1. Cation first, then anion • 2. Monatomic cation = name of the element • Ca 2+ = calcium ion • 3. Monatomic anion = root + -ide • Cl- = chloride • Ca. Cl 2 = calcium chloride

Naming Ionic Compounds (continued) Metals with multiple oxidation states some metal forms more than one cation • - use Roman numeral in name • - • Pb. Cl 2 • Pb 2+ is cation • Pb. Cl 2 = lead(II) chloride

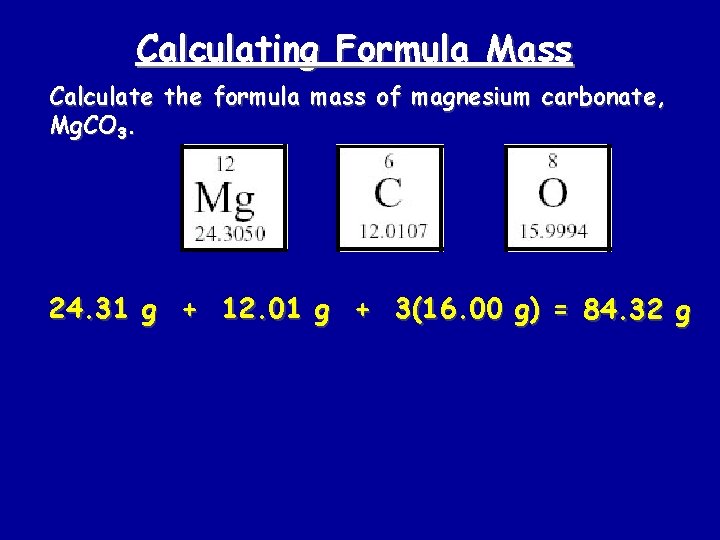
Calculating Formula Mass Calculate the formula mass of magnesium carbonate, Mg. CO 3. 24. 31 g + 12. 01 g + 3(16. 00 g) = 84. 32 g

Calculating Percentage Composition Calculate the percentage composition of magnesium carbonate, Mg. CO 3. From previous slide: 24. 31 g + 12. 01 g + 3(16. 00 g) = 84. 32 g 100. 00

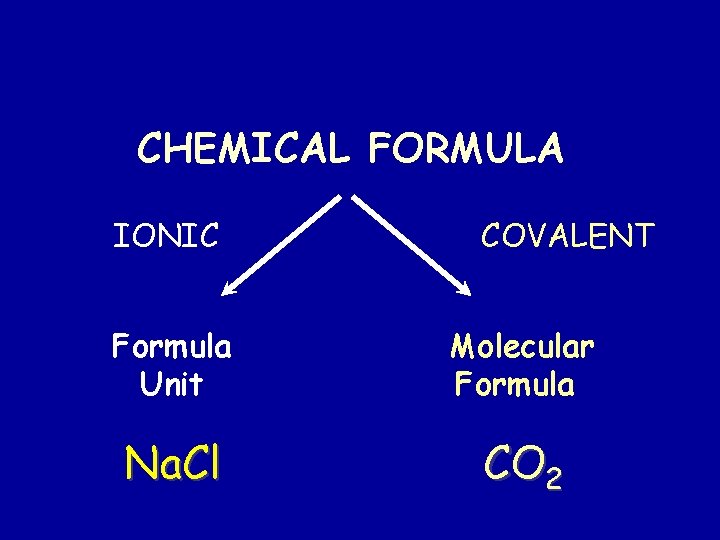
CHEMICAL FORMULA IONIC COVALENT Formula Unit Molecular Formula Na. Cl CO 2

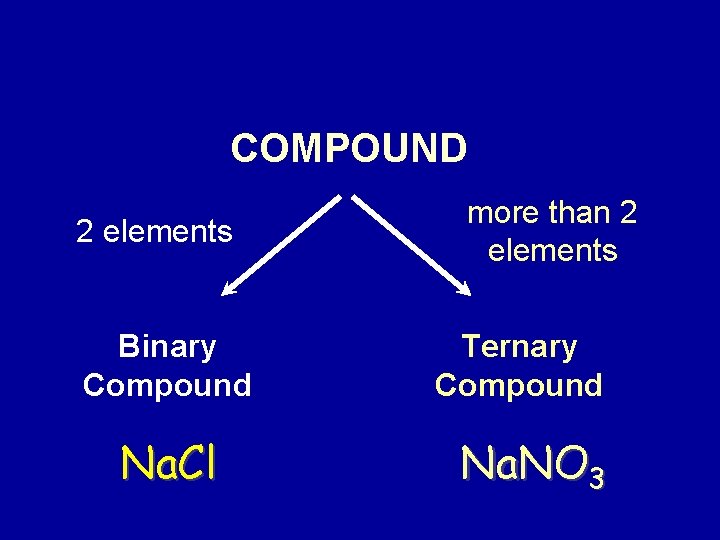
COMPOUND 2 elements Binary Compound Na. Cl more than 2 elements Ternary Compound Na. NO 3

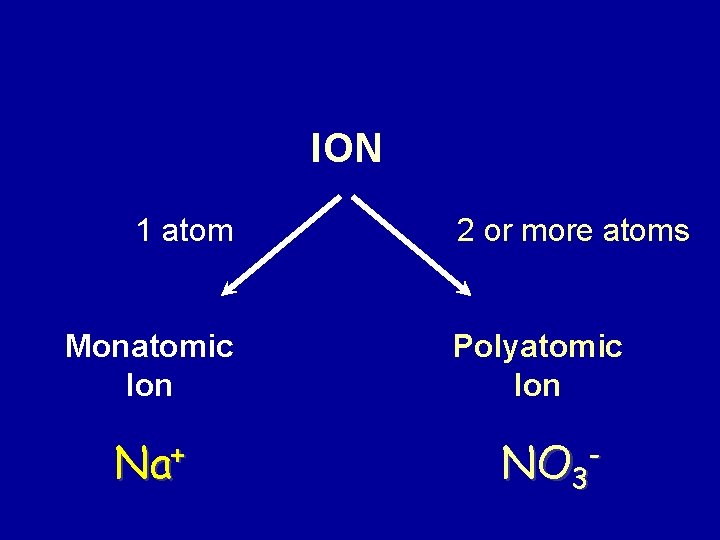
ION 1 atom Monatomic Ion Na+ 2 or more atoms Polyatomic Ion NO 3 -



Formulas Empirical formula: the lowest whole number ratio of atoms in a compound. Molecular formula: the true number of atoms of each element in the formula of a compound. q molecular formula = (empirical formula)n [n = integer] q molecular formula = C 6 H 6 = (CH)6 q empirical formula = CH

Formulas (continued) Formulas for ionic compounds are ALWAYS empirical (lowest whole number ratio). Examples: Na. Cl Mg. Cl 2 Al 2(SO 4)3 K 2 CO 3

Formulas (continued) Formulas for molecular compounds MIGHT be empirical (lowest whole number ratio). Molecular: H 2 O C 6 H 12 O 6 C 12 H 22 O 11 Empirical: H 2 O CH 2 O C 12 H 22 O 11

Empirical Formula Determination 1. Base calculation on 100 grams of compound. 2. Determine moles of each element in 100 grams of compound. 3. Divide each value of moles by the smallest of the values. 4. Multiply each number by an integer to obtain all whole numbers.

Empirical Formula Determination Adipic acid contains 49. 32% C, 43. 84% O, and 6. 85% H by mass. What is the empirical formula of adipic acid?

Empirical Formula Determination (part 2) Divide each value of moles by the smallest of the values. Carbon: Hydrogen: Oxygen:

Empirical Formula Determination (part 3) Multiply each number by an integer to obtain all whole numbers. Carbon: 1. 50 x 2 3 Hydrogen: 2. 50 x 2 5 Oxygen: 1. 00 x 2 2 Empirical formula: C 3 H 5 O 2

Finding the Molecular Formula The empirical formula for adipic acid is C 3 H 5 O 2. The molecular mass of adipic acid is 146 g/mol. What is the molecular formula of adipic acid? 1. Find the formula mass of C 3 H 5 O 2 3(12. 01 g) + 5(1. 01) + 2(16. 00) = 73. 08 g

Finding the Molecular Formula The empirical formula for adipic acid is C 3 H 5 O 2. The molecular mass of adipic acid is 146 g/mol. What is the molecular formula of adipic acid? 2. Divide the molecular mass by the mass given by the emipirical formula. 3(12. 01 g) + 5(1. 01) + 2(16. 00) = 73. 08 g

Finding the Molecular Formula The empirical formula for adipic acid is C 3 H 5 O 2. The molecular mass of adipic acid is 146 g/mol. What is the molecular formula of adipic acid? 3. Multiply the empirical formula by this number to get the molecular formula. 3(12. 01 g) + 5(1. 01) + 2(16. 00) = 73. 08 g (C 3 H 5 O 2) x 2 = C 6 H 10 O 4