Draw and label the structure of the atom
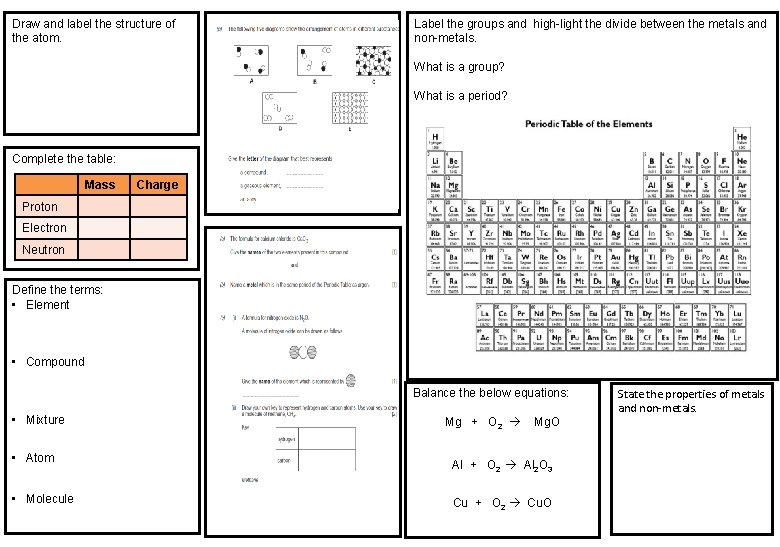
Draw and label the structure of the atom. Label the groups and high-light the divide between the metals and non-metals. What is a group? What is a period? Complete the table: Mass Charge Proton Electron Neutron Define the terms: • Element • Compound Balance the below equations: • Mixture • Atom • Molecule Mg + O 2 Mg. O Al + O 2 Al 2 O 3 Cu + O 2 Cu. O State the properties of metals and non-metals.

Write how many there are in: Electrons: Write the electron configuration of the following atoms: 1. Sodium Protons: Neutrons: What is an isotope? 2. Carbon 3. Chlorine 4. Neon 5. Lithium How many electrons are able to go in the 1 st, 2 nd, 3 rd and 4 th shell. The configuration tells us a number of things: 1. The number of electrons in the atom 2. The number on each of the shells. 3. The number of shells tell us the period the atom is found in. 4. The last number tells us the group it is in. Draw an electron shell diagram for sodium. State the number of electrons, neutron and protons found in Na, F, Cl, Mg and H. Add to the 5 elements above the period and the group it is found in. 6 QER
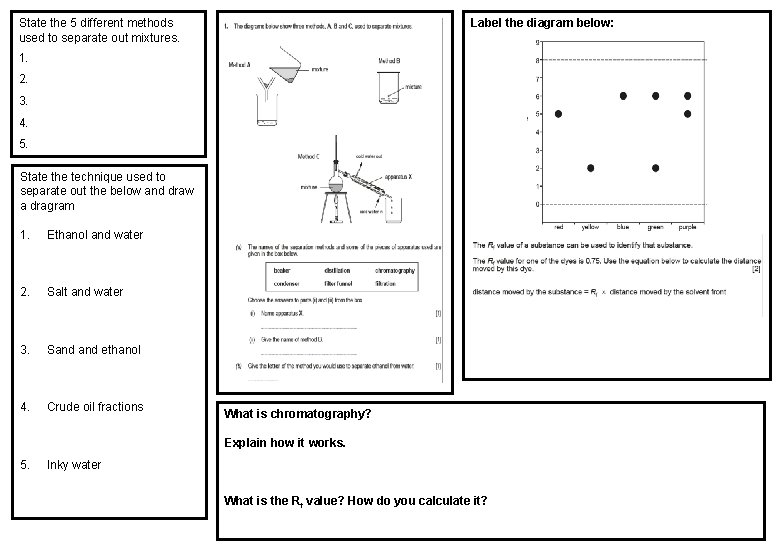
State the 5 different methods used to separate out mixtures. Label the diagram below: 1. 2. 3. 4. 5. State the technique used to separate out the below and draw a dragram 1. Ethanol and water 2. Salt and water 3. Sand ethanol 4. Crude oil fractions What is chromatography? Explain how it works. 5. Inky water What is the Rf value? How do you calculate it?

Write the formula for the following compounds: 1. Aluminium oxide 2. Iron (III) oxide 3. Calcium Chloride 4. Magnesium Bromide 5. Potassium Chloride Calculate the mass of copper: Calculate the percentage of impurity :
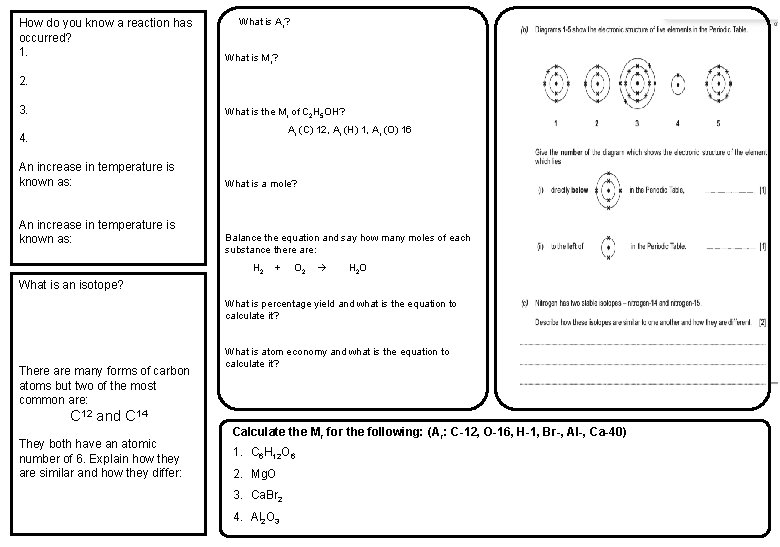
How do you know a reaction has occurred? 1. What is Ar? What is Mr? 2. 3. What is the Mr of C 2 H 5 OH? Ar (C) 12, Ar (H) 1, Ar (O) 16 4. An increase in temperature is known as: What is a mole? Balance the equation and say how many moles of each substance there are: H 2 + O 2 H 2 O What is an isotope? What is percentage yield and what is the equation to calculate it? There are many forms of carbon atoms but two of the most common are: What is atom economy and what is the equation to calculate it? C 12 and C 14 They both have an atomic number of 6. Explain how they are similar and how they differ: Calculate the Mr for the following: (A r: C-12, O-16, H-1, Br-, Al-, Ca-40) 1. C 6 H 12 O 6 2. Mg. O 3. Ca. Br 2 4. Al 2 O 3
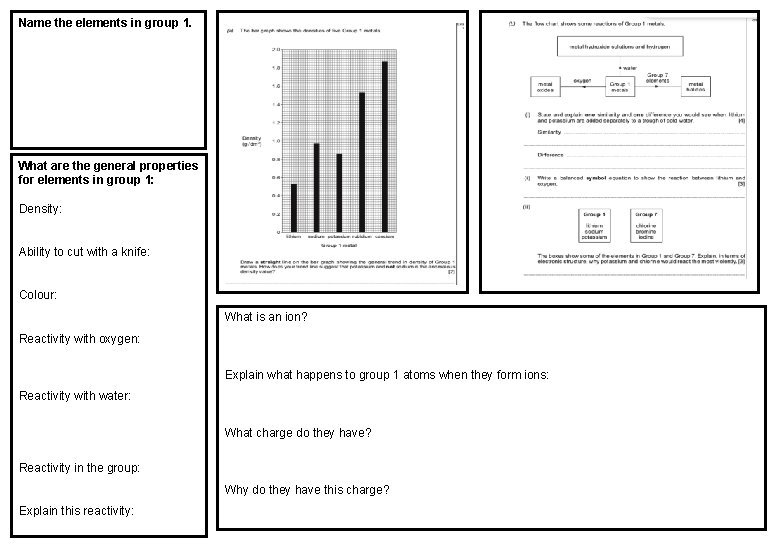
Name the elements in group 1. What are the general properties for elements in group 1: Density: Ability to cut with a knife: Colour: What is an ion? Reactivity with oxygen: Explain what happens to group 1 atoms when they form ions: Reactivity with water: What charge do they have? Reactivity in the group: Why do they have this charge? Explain this reactivity:
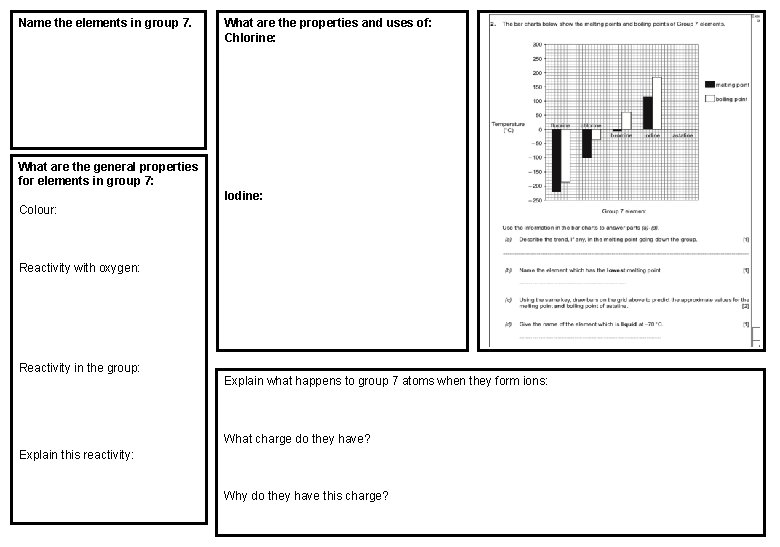
Name the elements in group 7. What are the properties and uses of: Chlorine: What are the general properties for elements in group 7: Iodine: Colour: Reactivity with oxygen: Reactivity in the group: Explain what happens to group 7 atoms when they form ions: What charge do they have? Explain this reactivity: Why do they have this charge?

Name the elements in group 0. What are the uses of: Helium: What are the general properties for elements in group 0: Colour: Neon: Reactivity in the group: Argon: Explain this reactivity:

Reaction with Iron: Group 1 metals (Alkali metals): Reaction of alkali metals with oxygen, water and halogens: Reaction with Alkali metal Chlorine Group 7 ( Halogens): Lithium What happens to the size of the atom as you go down the groups? How are the groups arranged? Sodium Potassium Bromine Iodine Oxygen Water

What is the test for: Hydrogen: Oxygen: How do you test for metal ions? Carbon Dioxide: What would you expect to see when testing the following metal ions: 1. Lithium (Li+) 2. Sodium (Na+) 3. Potassium (K+) How do you test for halogen ions? What would you expect to see when testing the following halogen ions: 1. Chlorine (Cl-) 4. Calcium (Ca 2+) 2. Sodium (Br-) 5. Barium (Ba 2+) 3. Potassium (I-)
- Slides: 10