Draft SA Marketing Code ERIC REURTS Marketing Code





- Slides: 10

Draft SA Marketing Code ERIC REURTS

Marketing Code Steering Committee PIASA - Pharmaceutical Industry Association NAPM – National Association of Pharmaceutical Manufacturers IMSA – Innovative Medicines of South Africa SMASA – Self Medication Manufacturers Association of South Africa PHARMISA – Pharmaceuticals made in South Africa IHD – International Healthcare Distributors LSPA – Logistics Service Providers Association (incl. NAPW & PHD) Supported by PSSA – Pharmaceutical Society of SA SAMED – South African Medical Devices Association SALDA – South African Laboratory and Diagnostics Association

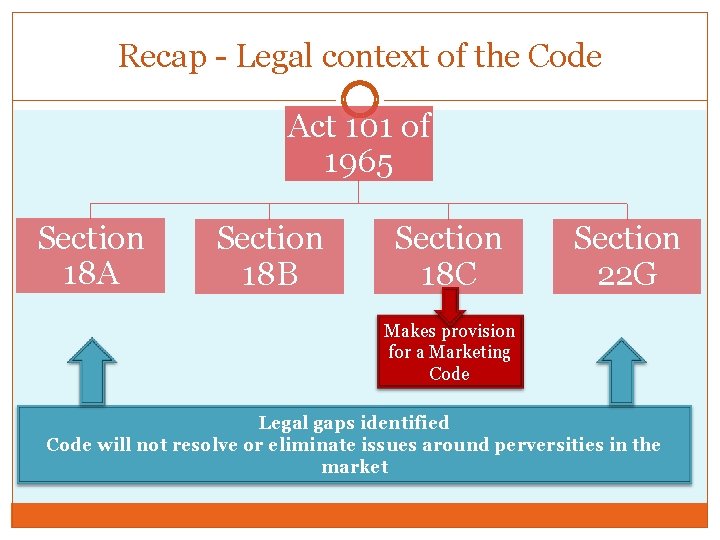
Recap - Legal context of the Code Act 101 of 1965 Section 18 A Section 18 B Section 18 C Section 22 G Makes provision for a Marketing Code Legal gaps identified Code will not resolve or eliminate issues around perversities in the market

2009 Code snapshot Major shift in enforcement model: From being regulated by Do. H to more self regulatory model Industry tasked with setting up MCA Independent board with broad representation Collaboration with ASA Outsourcing of code administration to ASA Do. H involvement Adherence to the Code still a condition of registration Breach of the Code is still a breach of the Act Interim Board appointed Function is drive to implementation of the Code Needed for governance purposes Steering Committee Interim board delegates tasks to steering committee

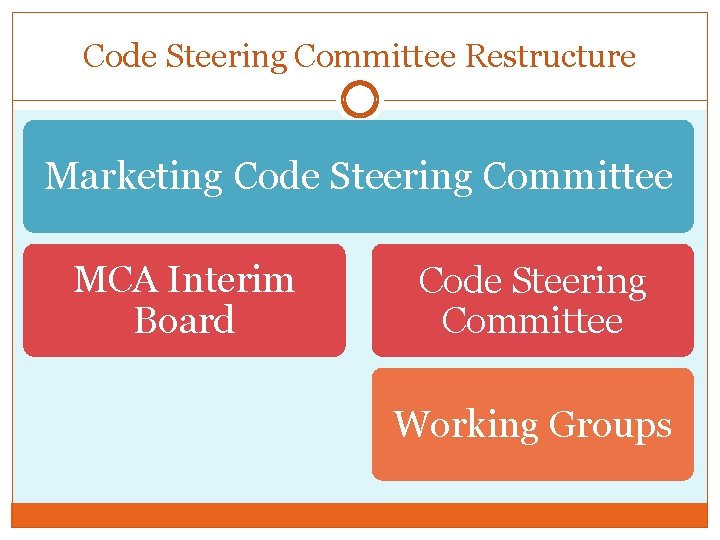
Code Steering Committee Restructure Marketing Code Steering Committee MCA Interim Board Code Steering Committee Working Groups

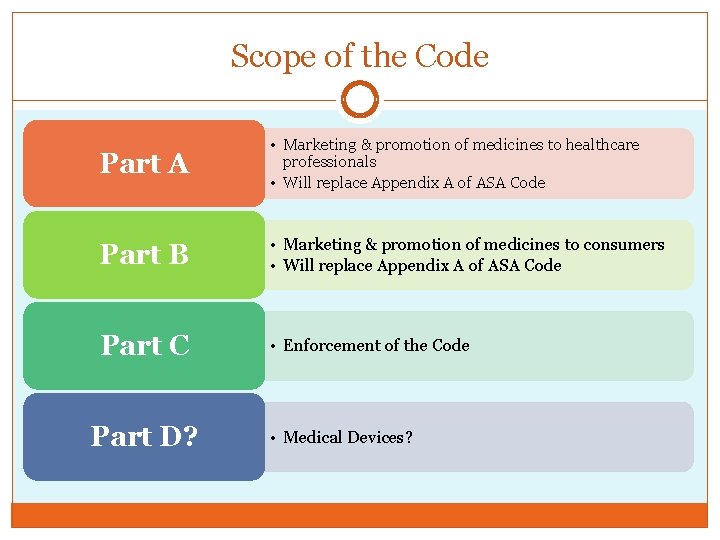
Scope of the Code Part A • Marketing & promotion of medicines to healthcare professionals • Will replace Appendix A of ASA Code Part B • Marketing & promotion of medicines to consumers • Will replace Appendix A of ASA Code Part C • Enforcement of the Code Part D? • Medical Devices?

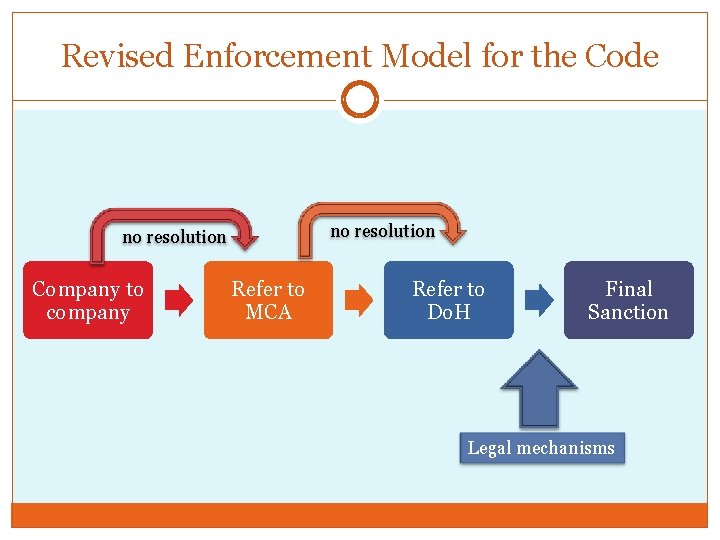
Revised Enforcement Model for the Code no resolution Company to company Refer to MCA Refer to Do. H Final Sanction Legal mechanisms

Some important questions Will the Code have ‘teeth’? YES! Section 18 C makes provision for a Code/s Regulations to Section 18 C will be published by Do. H Adherence to the Code will be a condition of registration When self regulatory mechanisms fail, complaints can be referred to Do. H Breach of the Code will be a breach of the Act Most severe sanction will be cancellation of registration of a product What about complementary medicines? Out of scope of the current SA Marketing Code HPA are developing their own Code

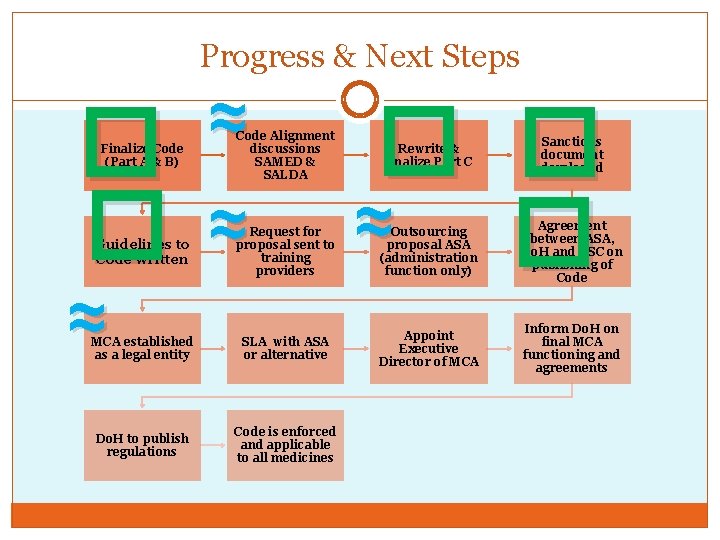
Progress & Next Steps � � Finalize Code (Part A & B) Guidelines to Code written ≈ ≈ Code Alignment discussions SAMED & SALDA ≈ Request for proposal sent to training providers MCA established as a legal entity SLA with ASA or alternative Do. H to publish regulations Code is enforced and applicable to all medicines � � ≈ � Rewrite & finalize Part C Sanctions document developed Outsourcing proposal ASA (administration function only) Agreement between ASA, Do. H and CSC on publishing of Code Appoint Executive Director of MCA Inform Do. H on final MCA functioning and agreements

Timelines to implementation A lot of work has been done already Process of implementation more complex than anticipated Initial timeline of June 2010 was set This timeline will not be met Certainty is that Code will be implemented by Q 3 2010