DR RUKHSANA KHALID DO YOU KNOW Water quality



































































- Slides: 77


DR. RUKHSANA KHALID

DO YOU KNOW? ?




Water quality refers to the physical, chemical, biological and radiological characteristics of water. It is the measure of the condition of water relative to the requirements of one or more biotic species and or to any human need or purpose.

WATER QUALITY INDICES Water quality indices for drinking water may be classified as follows: Guidelines recommended by WHO in 2011 for water quality relate to: 1. Acceptability aspects. 2. Microbiological aspects. 3. Chemical aspects.

1. ACCEPTABILITY ASPECTS: Safe drinking water should be: - Free from colour - Free from turbidity - Free from odour - Free from disagreeable taste

2. MICROBIOLOGICAL ASPECTS Ideally drinking water should not contain any micro-organisms known to be pathogenic. The primary bacterial indicator is the coliform group of organisms.

a. Coliform organisms: Include - all aerobes - facultative anaerobes - gram negative, non spore forming, motile & non motile rods capable of fermenting lactose.

b. Faecal streptococci: - It is present in faeces but in smaller amounts. - The finding of faecal streptococci in water is regarded as important confirmatory evidence of recent faecal pollution of water.

c. Cl. perfringens: - They are also present in faeces. - Their spores are capable of surviving in water for longer duration and resist chlorination at doses normally used in water works practice. - Their presence indicates faecal contamination at some remote time.

3. CHEMICAL ASPECTS a. Chlorides: All water contains chlorides. Since the chloride content of water varies from place to place, it is necessary to know the normal range of chloride, any excess above the normal range should raise suspicion of contamination. Normal value= 200600 mg/litre.

b. Hardness: Total hardness should not exceed 300 mg/litre. c. Free & Saline ammonia: This is an excellent indicator of sewage contamination of recent origin. Proteinaceous matter present in faecal matter is degraded and the resulting nitrogen is converted into bacterial action.

d. Albuminoid ammonia: It is measure of the decomposable organic matter, yet to be oxidized. Underground water should not contain albuminoid ammonia. In potable water it should not exceed 0. 1 mg/liter. e. Nitrites: It should be zero in potable water, their presence indicates recent contamination. however in deep well, it is present normally due to reduction of nitrates by ferrous salts. Water, except deep well, containing nitrites should be viewed with suspicion.

f. Nitrates: Nitrates tell the chemical story of past history of water. - their presence indicates old contamination, provided nitrites are absent. - levels should not exceed 1 mg/liter. g. Oxygen absorbed: The amount of oxygen absorbed by water is regarded as an approximate level of the amount of organic matter present in water. It should not be more than 1 mg/L in 3 hours at 37 deg. C.

h. Dissolved oxygen: Should not be less than 5 mg/L. i. Toxic Substances: Their presence above prescribed limits in water supply constitute grounds for rejection. They include - arsenic, cadmium, lead, mercury, cyanide and selenium.

POINT TO REMEMBER: Presence of ammonia, nitrites, nitrates & increase amount of oxygen absorbed indicates contamination.

4. RADIOLOGICAL ASPECTS There is evidence from both human and animal studies that radiation exposure at low to moderate doses may increase the longterm incidence of cancer. Animal studies in particular suggest that the rate of genetic malformations may be increased by radiation exposure.

Acute health effects of radiation, leading to reduced blood cell counts and, in very severe cases, death, occur at very high doses of exposure of the whole body or large part of the body. Due to the low levels of radionuclides typically found in drinking-water supplies, acute health effects of radiation are not a concern for drinking-water supplies.


WATER QUALITY STANDARDS WHO ‘ International Standards For Drinking Water’ relate to 5 water quality variables. 1. Microbiological pollutants. 2. Toxic substances. 3. Specific chemical substances that may affect health. 4. Characteristics affecting the acceptability of water. 5. Radioactive substances.

1. MICROBIOLOGICAL POLLUTANTS These are the standards relating to the presence of bacteria and viruses in drinking water. • STANDARD OF BACTERIAL QUALITY - Treated water- ideally all samples taken from distribution system should be free from coliform organisms. Following standards are recommended:

a. Throughout any year, 95% of samples should not contain any coliform organisms in 100 ml. b. No sample should contain E. coli in 100 ml. c. No sample should contain more than 10 coliform organisms per 100 ml. d. Coliform organisms should not be detectable in 100 ml of any two consecutive samples.

• STANDARD OF VIRAL QUALITY The WHO standards fix the limit for viruses at one plaque forming unit (PFU). A Plaque Forming Unit (PFU) is a measure of the particles capable of forming plaques per unit volume such as virus particles.

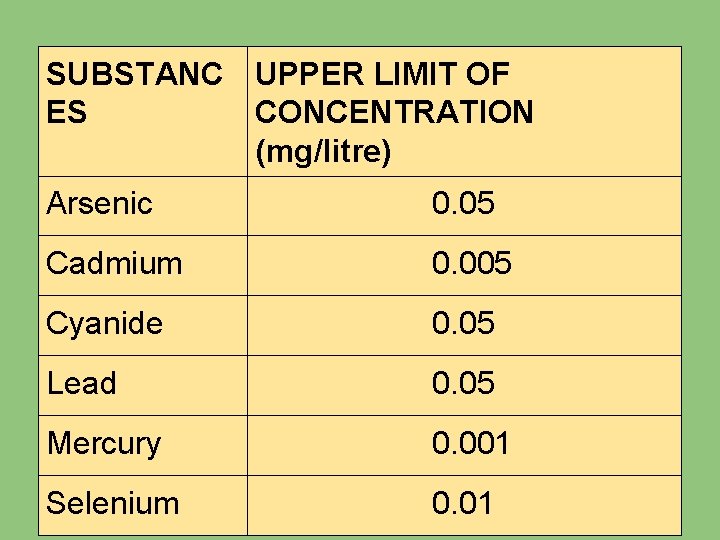
2. TOXIC SUBSTANCES The presence of following substances in excess of amount should constitute grounds for rejection of water as a public supply for domestic use:

SUBSTANC UPPER LIMIT OF ES CONCENTRATION (mg/litre) Arsenic 0. 05 Cadmium 0. 005 Cyanide 0. 05 Lead 0. 05 Mercury 0. 001 Selenium 0. 01

3. SPECIFIC SUBSTANCES THAT MAY AFFECT HEALTH A. FLUORIDES: Fluoride is essential constituent of drinking water. It should be more than 0. 5 mg/L. • DEFICIENCY: - deficiency causes dental caries • EXCESSIVENESS: - Excessive of fluorides in drinking water may give rise to dental fluorosis in children.

- When present in much higher amounts, it causes endemic cumulative fluorosis with resultant skeletal damage in both children and adults. B. NITRATES: Nitrates are dangerous to human health only in infants. - Excess amount can causes infantile methemoglobinemia.

C. AROMATIC HYDROCARBONS: Some polynuclear aromatic hydrocarbons (PAH) are known to be carcinogenic. • Concentration should not exceed 0. 2 microgram/L

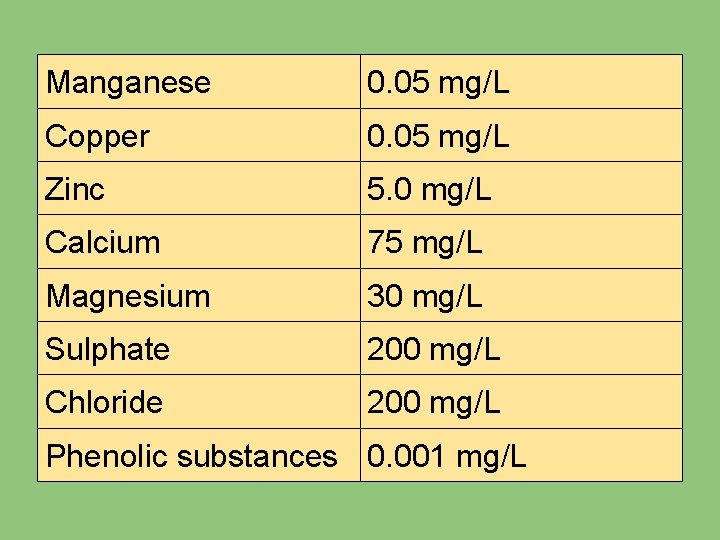
4. SUBSTANCE AFFECTING ACCEPTABILITY OF WATER The following criteria have been suggested by WHO in assessing the potability of water: SUBSTANCE p. H range HIGHEST DESIRABLE LEVEL 7. 0 - 8. 5 Total solids 500 mg/L Total hardness 2 m. Eq/L Iron 0. 1 mg/L

Manganese 0. 05 mg/L Copper 0. 05 mg/L Zinc 5. 0 mg/L Calcium 75 mg/L Magnesium 30 mg/L Sulphate 200 mg/L Chloride 200 mg/L Phenolic substances 0. 001 mg/L

5. RADIOACTIVE SUBSTANCES Pollution of water supplies by radioactive substances represents an increasing hazards with regard to water quality. - Radioactivity is expressed as picocuries per litre (p. Ci/L) WHO has proposed the following limits of radioactivity as acceptable: • Gross alpha activity 3 p. Ci/L • Gross beta activity 30 p. Ci/L


SURVEILLANCE OF DRINKING WATER QUALITY


Surveillance of drinking water is essentially a health measure. It is intended to protect the public from waterborne diseases. The elements of surveillance programme are: 1. Sanitary Survey 2. Sampling 3. Bacteriological surveillance 4. Biological examination 5. Chemical surveillance

1. SANITARY SURVEY: The sanitary survey is an on-the-spot inspection and evaluation of entire water supply system by a qualified person. PURPOSE: - To detect faults and correction of deficiencies.

2. SAMPLING: Proper water sampling is very important step in surveillance of water quality. It should be done thoroughly and carefully according to standards of WHO. Sampling should be done for: a. Physical & Chemical Examination b. Bacteriological Examination

a. SAMPLING FOR PHYSICAL & CHEMICAL EXAMINATION: • Sample should be collected in CLEAN GLASS STOPPERED BOTTLE made of neutral glass. • Capacity of the bottle should not be less than 2 litres. • Stoppered glass bottle technically known as “Winchester Quart Bottle” are suitable.

WINCHESTER QUART BOTTLE

• Before collecting the sample rinse the bottle well three times with the water filling it each time about ⅓ full. • Then fill it with water. • Tie the stopper tightly down with a piece of cloth over it. • Seal the string

b. SAMPLING FOR BACTERIOLOGICAL EXAMINATION: • It should be collected in clean sterilized bottle made of neutral glass of capacity 200 -250 ml and provided with a glass stopper having an overlapping rim. • The stopper must be relaxed by an intervening strip of paper to prevent breakage of bottle during sterlization or jamming of bottle.

• The stopper and neck of the bottle should be protected by a paper cover. • If the water to be sampled contains or is likely to contain chlorine, a small quantity of sodium thiosulphate (0. 1 ml of 3. 0 % solution) should be added to the bottle before sterilization. • Sterile sample bottles should be obtained from the laboratory which is to carry out the analysis. • The sampling bottle should not be opened until the moment at which it is required for filling.

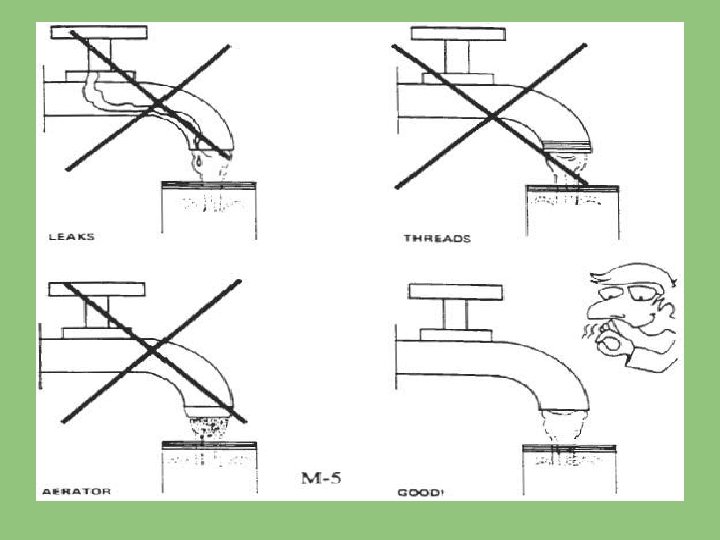
1. COLLECTION OF WATER FROM A TAP: • When a sample is to taken from as tap in regular use, the tap should be opened fully. • Water should be run to waste at least for 2 minutes in order to flush the interior of nozzle and to discharge the stagnant water in the service pipe.

• In case of the samples to be collected from taps which are not in regular use, tap should be sterilized by heating it either with a blow lamp or with an ignited piece of cotton soaked in methylated spirit, until it is unbearably hot to touch. • Then the tap should be cooled by allowing water to run to waste before the sample is collected.

• The bottle should be held near the base with one hand the stopper and paper cover it removed together and held in the fingers. • The sample bottle should be filled from a gentle stream of water from the tap.

THINGS NOT TO DO • Avoid splashing. • The collection of samples from taps which are leaky should be avoided because the water might run down the outside of tap and enter the bottle causing contamination. • If this cannot be avoided special precautions should be taken to clean the outside of tap and to flame it sufficiently to ensure sterility.


2. COLLECTION OF WATER FROM RIVERS, RESERVOIRS, LAKES, WELLS ETC • Samples from rivers and streams should not be taken too near the bank or too away from the point of draw off. • For collecting samples directly, a bottle with a string attached to the neck which if fully wrapped in paper and sterilized should be used.

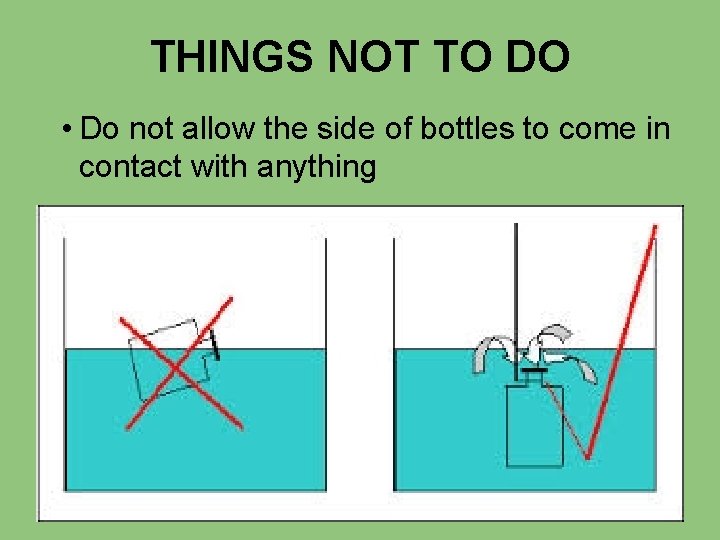
THINGS NOT TO DO • Do not allow the side of bottles to come in contact with anything

• Before taking the sample, paper cover should be removed. • Another long clean string should be tied to the end of the sterilized string and the bottle lowered into the water and allowed to fill up. • The bottle should be then raised and the stopper with cover replaced.

ANOTHER METHOD: • Hold the bottle by the bottom and plunge its neck downwards below the surface of water. • The bottle is then turned until the neck points slightly upwards, the mouth being directed towards the current. • If no current exists, as in a reservoir, a current should be artificially created by pushing the bottle horizontally forward in the direction away from the hand.

• When full bottle is raised rapidly above the surface and the stopper replaced. • If a sample is to be taken from a well fitted with a pump, the water should be pumped to waste for about 2 minutes and the sample collected from the pump delivery or from a tap on the discharge.

3. TRANSPORT & STORAGE OF SAMPLES: • The bacteriological examination of the sample should be commenced as soon as possible after collection. • When it is not feasible, the sample should be kept in ice until it is taken for analysis. • All such iced samples should be taken for analysis within 48 hours after collection.

LABELLING OF SAMPLE: Sample should be labelled with following particulars - Source of water - Date & Time of collection - Particulars of recent rainfall and findings of sanitary survey should also be supplied with the sample.


3. BACTERIOLOGICAL SURVEILLANCE: The tests usually employed in water bacteriology are: - Presumptive coliform test - Tests for the detection of faecal streptococci and Cl. perfringens - Colony count

• PRESUMPTIVE COLIFORM TEST: (I) MULTIPLE TUBE METHOD: This test is based on estimating the most probable number (MPN) of coliform organisms in 100 ml of water. • This test is carried out by inoculating measured quantities of the sample water (0. 1, 1. 0, 10, 50) into tubes of Mc. Conkey’s Lactose Bile Salt Broth with bromocresol as an indicator.

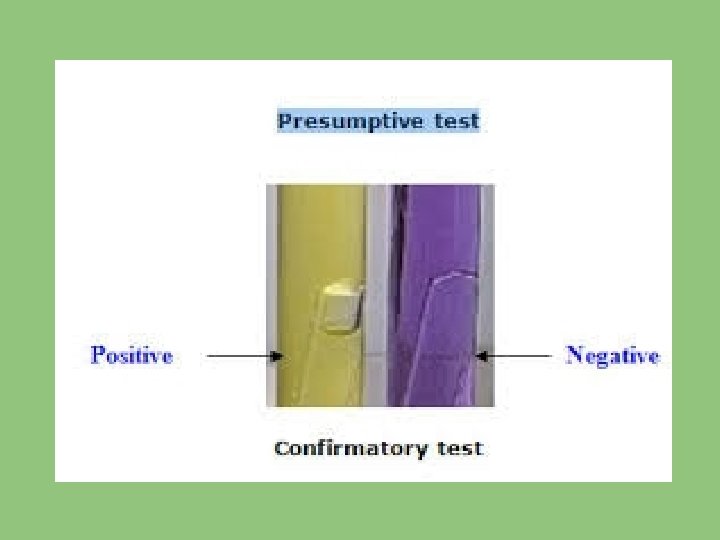
• The tubes are incubated for 48 hours. • From the number of tubes showing acid & gas, an estimate of MPN of coliform organisms in 100 ml of sample water can be obtained from statistical tables. This result is known as “Presumptive Coliform Count” The presumption being each tube showing fermentation contains coliform organisms.

CONFIRMATORY TEST: Next step is to confirm the coliform organisms in each tube showing positive presumptive reaction. Confirmation is required in case of chlorinated water. • Confirmation is done by subculturing each presumptive positive tube in 2 tubes of brilliant green bile broth, one is incubated at 37 deg C up to 48 hours for the confirmation of presence of coliform organism and the

other is incubated at 44 deg C and inspected after 6 and 24 hours to decide whether E. coli is present or not. • E. coli is the only coliform which is capable of producing gas from lactose at 44 deg. C


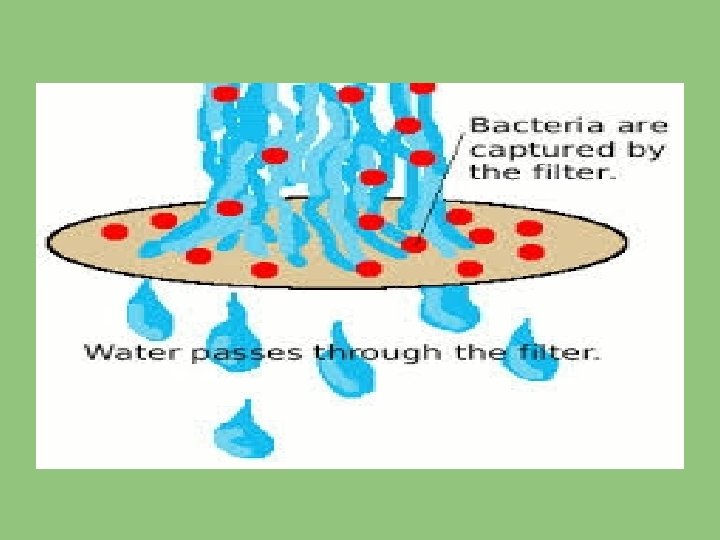
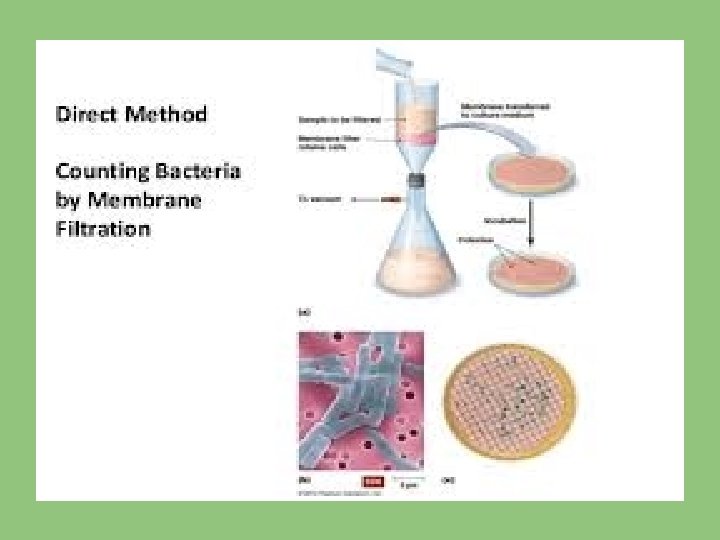
(II) MEMBRANE FILTRATION TECHNIQUE: In some countries it is used, measured volume of sample is filtered through a membrane made up of cellulose ester. All the bacteria present in water are retained on the surface of membrane and by inoculating the membrane face upwards on suitable media and at appropriate temperature.



ADVANTAGE OVER MULTIPLE TUBE METHOD: It is possible to count the colonies and obtain results within 20 hours as compared to 72 -96 hours required for the usual multiple tube method.

• THE DETECTION OF FAECAL STREPTOCOCCI AND CL. PERFRINGENS: The presence of faecal streptococci and cl. perfringens provides useful confirmatory evidence of the faecal pollution of water in doubtful cases.

• COLONY COUNT: Colony counts on nutrient agar at 37 deg. C and 22 deg. C are frequently used in the bacteriological examination. • Colony counts provide an estimate of the general bacterial purity of water. • A single count is of little value but counts from same source at frequent intervals may be of considerable value.

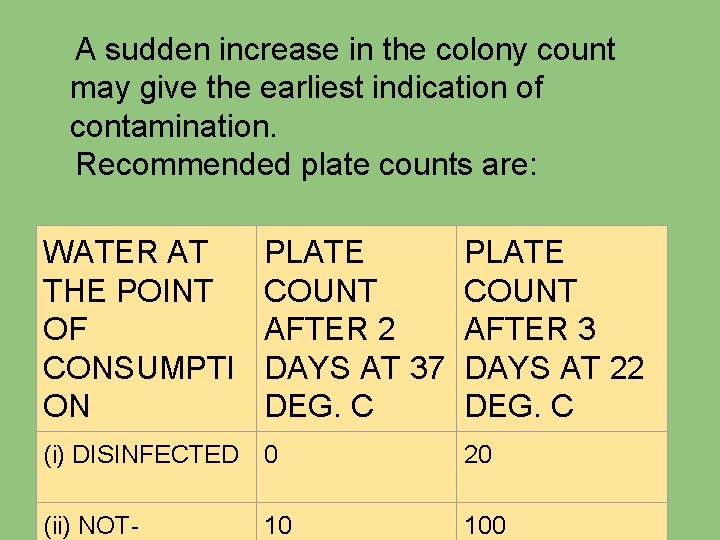
A sudden increase in the colony count may give the earliest indication of contamination. Recommended plate counts are: WATER AT THE POINT OF CONSUMPTI ON PLATE COUNT AFTER 2 DAYS AT 37 DEG. C PLATE COUNT AFTER 3 DAYS AT 22 DEG. C (i) DISINFECTED 0 20 (ii) NOT- 10 100

4. BIOLOGICAL EXAMINATION: Water may contain microscopic organisms such as algae, fungi, yeast, protozoa etc. These organisms are collectively called as “plankton”. - Plankton organisms produce objectionable tastes and odour. - They are an index of pollution. - Degree of pollution is assessed quantitatively & qualitatively by noting the

type and number of organisms prevailing in water. 5. CHEMICAL SURVEILLANCE: Chemical surveillance of drinking water is assuming greater importance in view of industrial and agricultural pollutants.

BASIC TESTS: Followings tests are done: - test for p. H - colour - turbidity - chlorides - ammonia - chlorine demand - residual chlorine

- tests for iron - tests for manganese - for toxic metals - pesticides - organic chemicals - radioactivity


THANK YOU. . !!